Senior leaders are rarely pulled into submissions because teams are behind schedule. They are pulled in when outcomes become uncertain—and when submission predictability in pharma breaks down.
When predictability fails, leaders are accountable for decisions made under compressed timelines, incomplete information, and heightened regulatory risk. Late-stage questions, unclear regulatory impact, and inconsistent explanations force escalation at precisely the moment when time, options, and tolerance for ambiguity are limited. What appears to be an execution problem is a breakdown in predictability—and predictability is a leadership responsibility, not an operational afterthought.

Yet many organizations still frame submission readiness as a downstream activity, something addressed during document assembly and final review. From a leadership perspective, this framing is backwards. By the time content reaches final review, predictability has already been won or lost. What happens next is not control; it is damage containment.
To achieve submission predictability in pharma, uncertainty must be identified, governed, and resolved earlier—before escalation becomes inevitable.
CMC Is Evaluated Twice — and the Second Evaluation Is the Real Test
CMC content is evaluated twice.
First, at the point of submission and approval.
Second, every time the product, process, site, specification, or control strategy changes post-approval.
Initial approval confirms that commitments are acceptable as submitted. Lifecycle review determines whether those commitments remain coherent under change within the approved framework .
Most organizations implicitly treat the first evaluation as the regulatory hurdle. Regulators behave differently. For them, lifecycle review is the real test: can the sponsor demonstrate that quality knowledge remains authoritative as it evolves?
Predictability breaks down when organizations prepare for point-in-time approval but are structurally unprepared for lifecycle interrogation.
When Uncertainty Surfaces, Leadership Gets Pulled In
Unpredictable submissions are not random events. They are escalation signals that unmanaged risk exists upstream and has gone undetected until deadlines compress and tolerance for ambiguity disappears.
From a leadership perspective, escalation rarely occurs because teams are unaware of requirements or working inefficiently. Escalation occurs when teams cannot confidently answer questions regulators are likely to ask about consistency, change impact, or alignment across submissions and markets—and there is no clear path to resolution.
In CMC, this pressure most often surfaces during variation work.
Whether triggered by:
- A site addition
- A scale change
- An analytical method update
- A specification adjustment
- A control strategy refinement
The underlying challenge is the same: the organization must prove that existing commitments remain valid, bounded, and internally consistent under change .
At that point, uncertainty becomes visible. Issues that could have been resolved quietly earlier now require senior judgment, risk tradeoffs, and external positioning. Leadership attention shifts from governance to intervention.
Predictability, therefore, is not about moving faster. It is about knowing, in advance, how governed CMC commitments will behave when they are reused, challenged, or modified—and being able to demonstrate that knowledge with confidence.
Predictability Is a Signal of Control, Not Effort
Organizations with predictable submissions are not simply better organized or more disciplined. They understand how their CMC content is reused, how it changes, and where regulatory questions are likely to surface—and they have mechanisms to govern that behavior.
Organizations with unpredictable submissions do not lack effort. They lack upstream control.
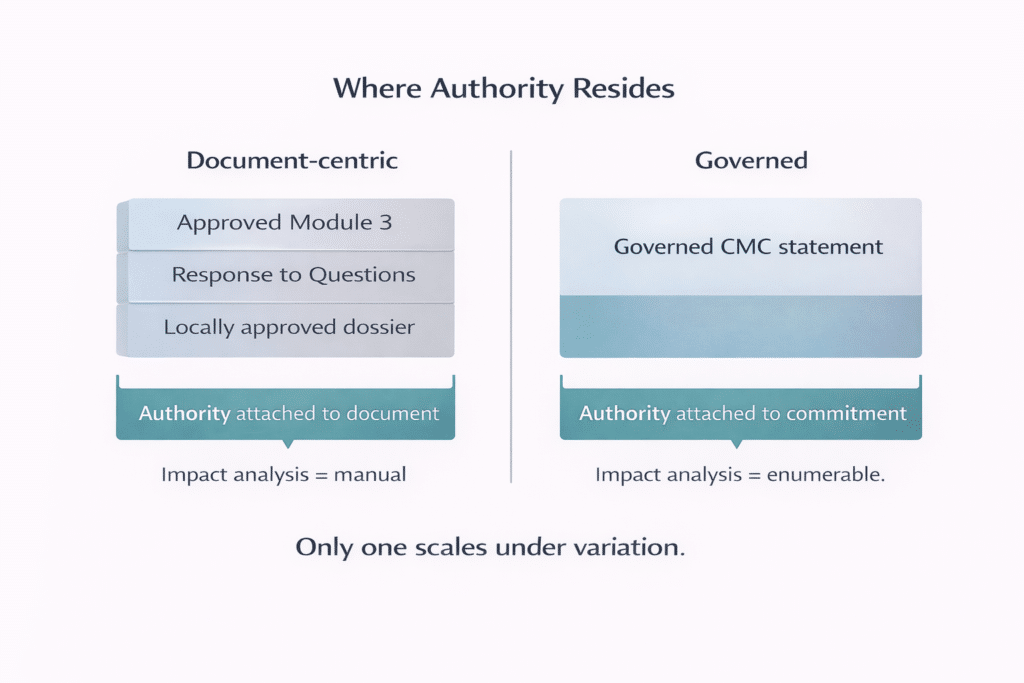
In document-centric environments, authority resides in assembled artifacts: the last approved Module 3 section, the most recent response to questions, the latest locally approved dossier. In practice, authority becomes dispersed across versions of the same underlying statement, each subtly adapted without a single authoritative source .
When timelines are long, these gaps remain hidden. When timelines compress—particularly during variations—they surface suddenly, and leadership escalation follows.
Why Leaders Get Pulled in Late (and Why That Matters)
Senior leaders are rarely drawn into submissions because teams are behind schedule. Delays are familiar and usually manageable. Leaders get pulled in when teams cannot explain outcomes with confidence.
Questions such as:
- Where else does this statement govern?
- Under what site, scale, or lifecycle conditions is this commitment valid?
- What downstream commitments are affected if this section changes?
- Why does this specification rationale diverge from what was previously approved?
When answers are unclear, escalation is the only remaining control mechanism. At that point, the organization is no longer managing readiness; it is managing exposure.
This pattern is structural. In document-centric models, assurance is rebuilt manually for each submission through expanded reviews and reconciliation. There is no defined escalation point earlier in the process—only a late-stage collision between uncertainty and deadlines.
The result consumes leadership bandwidth without improving predictability, resolving individual issues while leaving the underlying system unchanged.
The Hidden Cost of Late-Stage Assurance
Late-stage assurance often feels responsible. More review, more alignment meetings, and more sign-offs create the impression of control. In practice, this approach carries organizational costs that compound over time.
Escalation risk increases as issues surface when options are limited and tradeoffs are more consequential. Regulatory posture weakens as responses rely increasingly on narrative reconciliation rather than demonstrable control over reusable CMC commitments. Leadership attention is diverted from strategic oversight to tactical decision-making under pressure. Organizational confidence erodes as teams normalize rework, reconciliation, and last-minute clarification as “just how submissions work.”
Over time, this pattern institutionalizes unpredictability. What should be treated as a preventable signal becomes an accepted operating condition.
Why This Matters Now
Regulatory direction is no longer limited to digitizing documents. Initiatives such as FDA’s PQ/CMC and KASA signal a shift toward structured, comparable, and lifecycle-aware representations of quality information .
Review is moving from reading CMC to interrogating it.
As agencies increase their ability to compare structured elements across strengths, applications, and post-approval changes, tolerance for ambiguity in how commitments are expressed shrinks. Narrative reconciliation becomes less sufficient. Inconsistency becomes visible at the data level rather than correctable at the prose level .
Organizations that continue to manage authoritative CMC knowledge exclusively as assembled documents will face repeated reconstruction, reconciliation, and revalidation—precisely the downstream burden structured review initiatives are designed to reduce .
Submission predictability in pharma does not start at publishing. It starts where CMC commitments are authored, reused, and governed—or not governed.
Why Review Alone Cannot Create Predictability
Review remains essential, but it does not scale as a primary control mechanism. Documents do not expose relationships between commitments. They obscure reuse, lineage, and change impact.
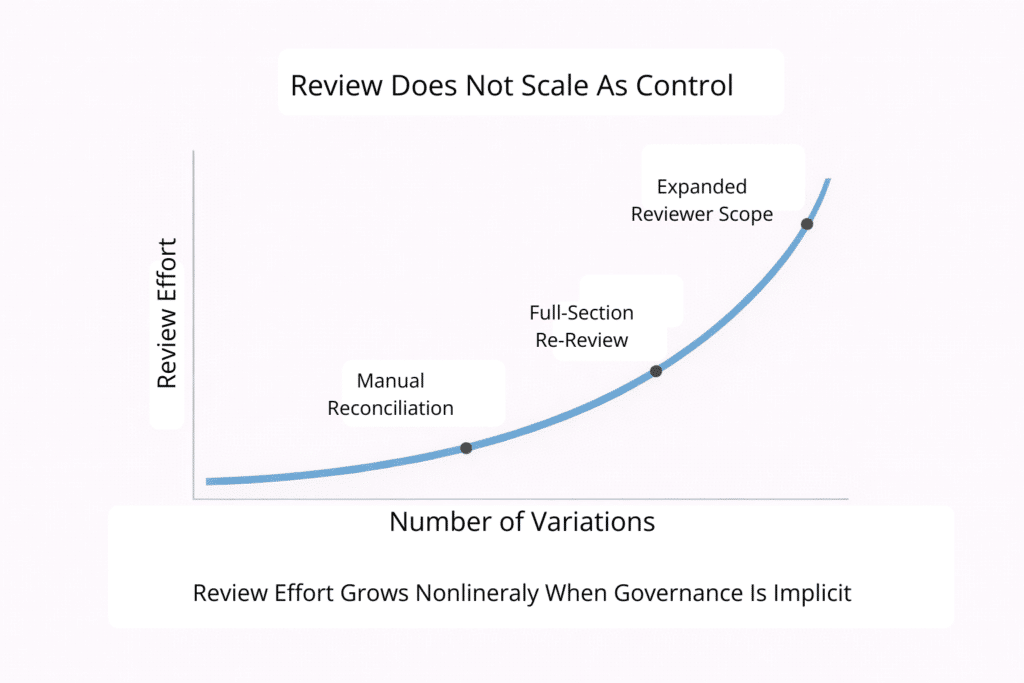
As portfolios grow and global filings proliferate, reviewers are forced to rediscover context repeatedly. Each cycle depends on memory, comparison, and manual searching. Review effort increases nonlinearly, while confidence improves only marginally.
Structure alone does not fix this.
Modularizing Module 3 content without relocating authority increases throughput. Throughput increases propagation. Propagation without explicit statement-level governance increases the cost of uncertainty .
Authority must relocate from the assembled document to the reusable statement.
When approval governs the document, reuse must be re-proven repeatedly. When approval governs the statement itself—with explicit scope, applicability, and lifecycle conditions—reuse becomes bounded and inspectable.
That shift is what changes the mechanics of variation work.
Governing Content Upstream Changes the Outcome
When CMC content is governed at the source, escalation patterns change.
Approved commitments exist as controlled statements rather than isolated text embedded in documents. Reuse is explicit. Validity conditions—site, scale, lifecycle stage—are defined and inspectable. Change impact becomes enumerable rather than inferred .
For leaders, this directly addresses the escalation problem. Fewer issues surface late because uncertainty is resolved earlier. Leadership involvement shifts from intervention to oversight. Timelines stabilize because submissions are assembled from governed knowledge rather than reconstructed artifacts. Regulatory responses strengthen because teams can show lineage, reuse, and bounded applicability with confidence.
Predictability becomes a property of the operating model—not the result of heroic effort.
AI Does Not Create Control, It Exposes It
As AI enters CMC content operations, this distinction becomes sharper.
AI accelerates whatever control model already exists.
In document-centric environments, acceleration increases output without increasing provability. Risk scales faster than review capacity.
In governed environments, AI becomes valuable precisely because it is constrained. It can retrieve approved statements, enumerate impact, surface divergence, and recommend bounded reuse—acting as an assistant to provability rather than a generator of unverified content .
Acceleration without governance increases risk.
Acceleration within governed bounds increases predictability.
How Docuvera Enables Predictable Readiness
Docuvera addresses the leadership escalation problem by relocating authority to governed CMC statements.
Instead of treating submissions as one-time document builds, Docuvera treats durable CMC commitments—specifications, control strategies, manufacturing descriptions, stability claims—as reusable, traceable regulatory objects with explicit scope and lifecycle control.
Approved statements carry metadata defining applicability and validity conditions. Change impact can be systematically enumerated. Reuse is bounded rather than assumed. Impact analysis shifts from best effort to system behavior .
Governance is embedded into authoring and review workflows. Content remains human-readable, but predictability is engineered into the system—giving leaders durable visibility and control across submissions, variations, and lifecycle change.
Next Steps
Identify where predictability is being rebuilt manually today. Assess where leadership intervention is most frequent and why. Then consider what changes when CMC content is governed upstream with Docuvera—before uncertainty surfaces, escalation begins, and leadership attention is pulled into avoidable risk.
Get in touch with our team to learn more.
Frequently Asked Questions: Submission Predictability in Pharma
Endnotes
- International Council for Harmonisation (ICH). ICH Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management. https://www.ich.org/page/quality-guidelines
- U.S. Food and Drug Administration. Pharmaceutical Quality–Chemistry, Manufacturing, and Controls (PQ-CMC) Initiative. https://www.fda.gov/drugs/pharmaceutical-quality-resources
- European Medicines Agency. Digital transformation and regulatory science strategy. https://www.ema.europa.eu
- McKinsey & Company. Future RegTech and the potential of generative AI in regulatory submissions (Jan 2024). https://www.mckinsey.com
- Gartner. Structured Content Authoring in Life Sciences Regulatory Operations. https://www.gartner.com