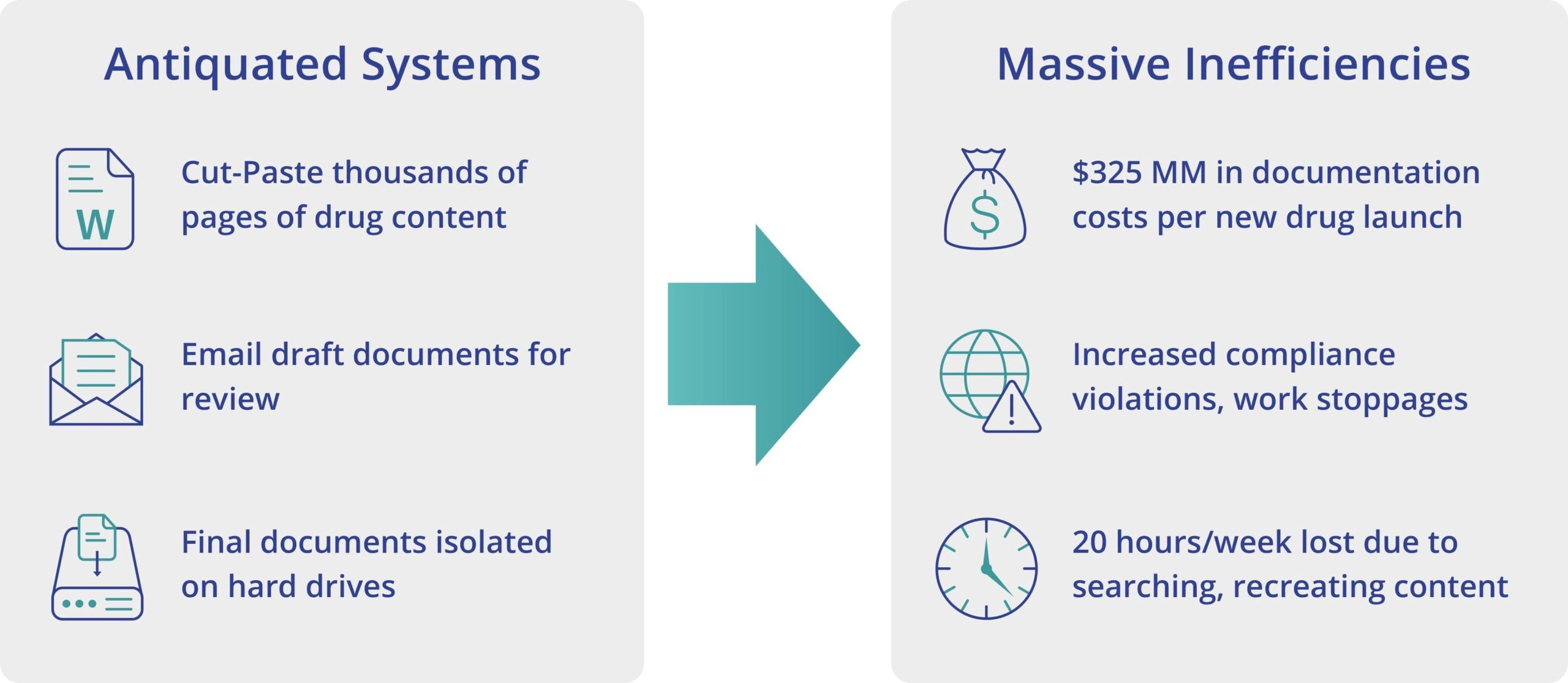
Antiquated systems contribute to the high cost of producing life sciences documentation, compliance violations, and missed deadlines.
For marketed drugs, information must be managed and updated with the associated product information throughout the entire product lifecycle. Maintaining this information worldwide is a complex challenge because core product information is often duplicated across thousands of documents. Then, as information grows and changes over time, manual business processes and technologies increase the risk of errors and delays in completing critical documentation.
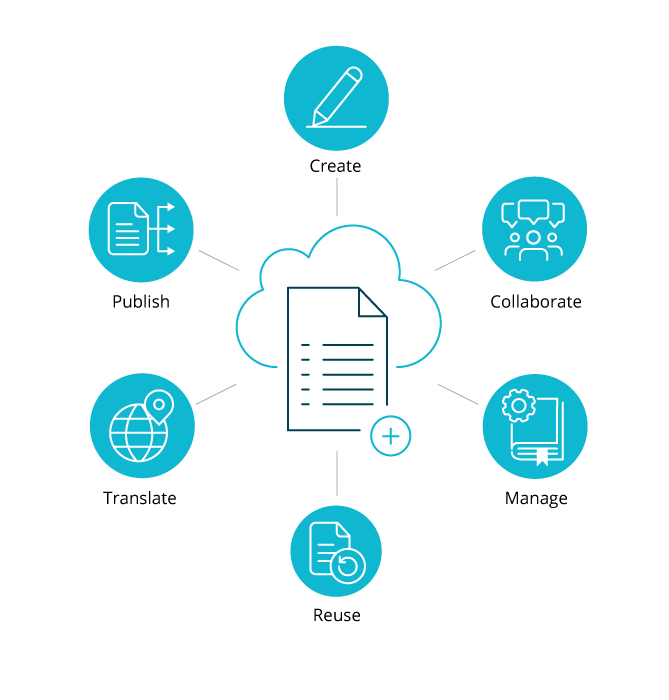
Across the drug development lifecycle, there are ample opportunities for information reuse from clinical all the way through to product commercialization.
That’s where Docuvera comes in.
Docuvera helps companies increase efficiency and output across all stages of pharmaceutical documentation.
See how Docuvera can work for you.
Clinical
Docuvera brings efficient and easy-to-use component-based authoring capabilities to the creation of and revision process for pre-clinical and clinical content.
Global Labeling
Docuvera’s end-to-end solution reduces labeling content production times, ensures alignment to compliant content, and creates faster time-to-market for regulatory submissions.
Medical Information
Docuvera reduces medinfo content production times and enables omnichannel distribution of medical content to health care professionals.
Chemistry Manufacturing and Controls
Docuvera enables the efficient creation and maintenance of CMC documentation by reusing core component content to assemble local documentation.
Aggregate Reporting and Safety Writing
Docuvera brings component-based reuse to the creation and maintenance of aggregate safety reports for all stages of the product lifecycle.
Quality Documents/SOPs
Review, approve, translate, reuse and publish quality documents and reuse approved content components to efficiently assemble accurate SOPs with Docuvera.
Looking for Technical Publications, Training and eLearning solutions?
Our sister solution, Author-it, enables authors to create, collaborate, manage, reuse, publish and translate documentation – all in one platform.