In the pharmaceutical industry, regulatory and medical writing teams face increasing content complexity that often outpaces their ability to scale. Global product launches, evolving regulatory mandates such as ePI (electronic Product Information) and IDMP (Identification of Medicinal Products), and the growing need for region-specific document variations have turned content creation and maintenance into significant operational challenges and compliance risks. Content creators—including regulatory writers, medical writers, and other stakeholders—face challenges in developing, editing, and publishing compliant content.
Structured content authoring (SCA) offers a powerful solution by breaking documents into modular, reusable blocks. This modularity enables teams to efficiently manage and update information across multiple projects and products. Structured content authoring also allows organizations to create content efficiently by providing user-friendly tools.
In contrast, unstructured content lacks proper organization, making it difficult to reuse, manage, and adapt—especially in complex, enterprise environments. Taking this a step further, AI-powered structured content authoring (AI-SCA) integrates artificial intelligence to enhance every stage of the content lifecycle—improving accuracy, accelerating approvals, and enabling smarter reuse of content components.
This article explores seven real-world use cases where AI-SCA is already transforming regulatory content management for pharmaceutical organizations, helping teams deliver compliant, up-to-date documentation faster and with greater confidence. As organizations embark on the structured content journey, they typically progress from initial content modeling to comprehensive structured content adoption, aligning teams and workflows along the way.
1. Global Labeling Automation
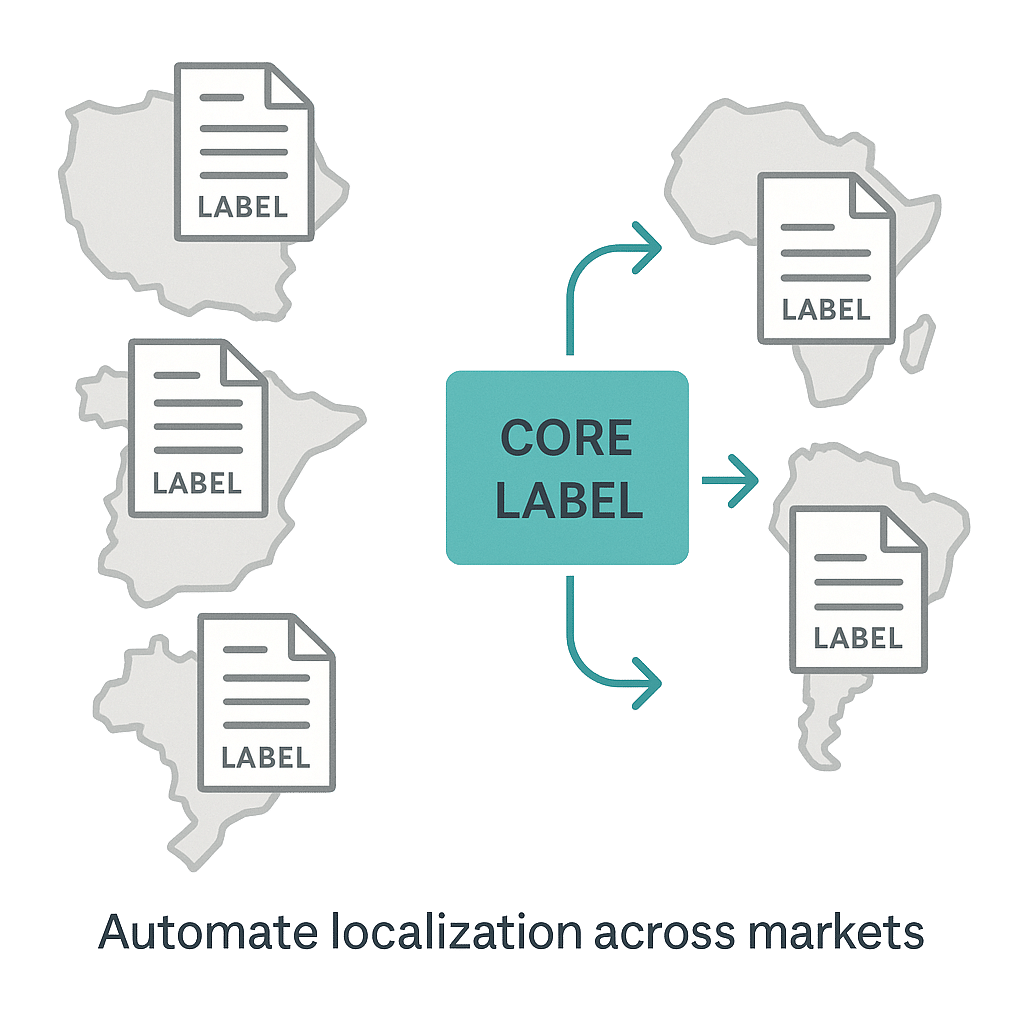
Labeling is one of the most highly regulated and complex areas of pharmaceutical documentation. Managing product labels across dozens of countries—each with unique formatting, language, and legal requirements—creates a massive challenge for content teams. Errors in labeling can lead to regulatory questions, submission delays, or even product withdrawals.
AI-powered structured content authoring helps by automating the localization process. It applies predefined rules to:
- Structure and adapt content modules per market
- Compare local labels against core data sheets (CCDS)
- Flag discrepancies automatically
Additionally, AI can recommend updated content blocks based on historical approval trends and feedback, ensuring that labeling stays accurate, compliant, and consistent across multiple platforms and languages.
2. Smart Reuse of Core Content Across Submissions
One of the major benefits of structured content is the ability to reuse content components efficiently. However, manual reuse risks pulling outdated or non-compliant versions of modules, leading to errors and wasted effort.
AI enhances this process by:
- Identifying the ideal content blocks based on document type, product specifics, and regulatory history
- Evaluating confidence levels of modules through prior approvals, updates, and audit trails
- Advising on reuse eligibility across countries with similar regulatory requirements or language standards
This enables regulatory teams to trust that they are applying the same content consistently, reducing duplication, accelerating submission readiness, and improving overall content quality.
3. Drafting Safety Narratives with Machine Assistance
Safety narratives are critical and resource-intensive documents during clinical trials. AI-powered structured content platforms can:
- Generate first drafts by pulling structured trial data into pre-defined narrative templates
- Recommend phrasing consistent with prior submissions
- Detect contradictions, such as mismatches between adverse event reports and medical history fields
By automating these initial drafts, teams report 30–40% time savings, freeing writers to focus on higher-value tasks such as data analysis and narrative refinement. This use case exemplifies how AI-SCA can improve both content creation efficiency and quality in pharma regulatory writing.
4. Metadata and Compliance Tagging at Scale

Metadata is the backbone of structured content management. Without accurate and comprehensive tagging, content modules cannot be effectively filtered, reused, or validated. However, manually tagging decades of regulatory documents is slow, labor-intensive, and prone to errors.
AI accelerates metadata tagging by:
- Automatically assigning tags based on phrasing, usage history, and document context
- Identifying missing or conflicting attributes before content reuse
- Learning from tagging patterns to suggest more accurate and compliant metadata sets
This ensures that content components are properly classified, making them easier to manage and maintain across multiple platforms and regulatory submissions.
5. Generating SmPCs and Regional Adaptations
Summary of Product Characteristics (SmPCs) and product information leaflets share common structures across regions but must be customized to meet local regulatory expectations around language, sequencing, and emphasis.
AI-powered SCA simplifies this process by:
- Pulling from master templates to generate country-specific variants automatically
- Adjusting phrasing based on regulatory preferences
- Cross-referencing sections to ensure internal consistency
This reduces duplication of effort while supporting local regulatory alignment and compliance across different languages and channels.
6. Accelerating Protocol and Investigator Brochure Development
Clinical study protocols and investigator brochures contain many standardized sections that are reused across studies and therapeutic areas. Manual reuse can introduce inconsistencies that complicate regulatory review.
AI-powered structured content platforms:
- Suggest approved components for similar study designs
- Track updates to scientific rationales across programs
- Reduce variation in repeated regulatory phrasing
This results in more consistent, accurate documentation and accelerates the development process, ensuring teams can deliver high-quality content aligned with user needs and regulatory standards.
7. Managing Structured Content and Reconciling Content Across CTD Modules
Regulatory submissions often duplicate or overlap content between modules of the Common Technical Document (CTD). For example, product quality data in Module 3 must align precisely with summaries in Module 2. Manually identifying inconsistencies is challenging and time-consuming.
AI platforms:
- Cross-analyze structured data across CTD modules
- Alert authors to discrepancies in formulation, specifications, or references
- Provide traceability back to source modules and historical submissions
This strengthens data integrity and compliance throughout the entire submission package, reducing risk and improving regulatory confidence.
Why These Use Cases Matter
These seven use cases demonstrate how AI-powered structured content authoring transforms regulatory operations from reactive, labor-intensive processes into proactive, insight-driven workflows. By automating repetitive tasks, improving metadata management, and enabling smarter content reuse, AI-SCA helps pharmaceutical teams deliver faster, more accurate, and compliant documentation across multiple channels and platforms.
If your organization is still manually drafting high-volume content, struggling with inconsistent reuse, or facing challenges in metadata tagging and content traceability, it’s time to consider integrating AI-powered structured content authoring into your content management system.
Where Docuvera Fits In
Docuvera empowers life sciences organizations to operationalize AI-powered structured content authoring without disrupting existing workflows. Designed specifically for regulatory complexity, Docuvera offers:
- Built on structured content: Component-based authoring with metadata governance from the ground up
- AI-enhanced capabilities: Auto-tagging, smart reuse, predictive validation, and draft generation
- Regulatory alignment: Ready for ePI, IDMP, CTD modernization, and global compliance mandates
- Seamless integration: Connects smoothly with Regulatory Information Management (RIM), Document Management Systems (DMS), and submission platforms
With Docuvera, regulatory teams gain immediate efficiency and long-term readiness for evolving global regulatory requirements, ensuring content stays up to date and compliant across all digital platforms.
The Bottom Line
Pharmaceutical companies can no longer afford to treat regulatory documentation as a purely manual exercise. By combining structured content authoring with AI, regulatory teams gain speed, accuracy, and operational resilience, building a foundation for digital compliance now and in the future.
Docuvera is helping leading organizations take this crucial step today, enabling them to meet tomorrow’s regulatory challenges with confidence and agility.
Connect today to learn more about how our modern SCA solution can optimize your labeling process.