How Architecture, Governance, and AI Are Reshaping Regulatory Work and Compliance
In the life sciences industry, structured content means organizing information into defined, reusable components that can be managed, updated, and published across multiple channels. Instead of treating text as long, unbroken blocks, organizations deconstruct information into its smallest meaningful parts. This enables the creation of diverse content types—ranging from clinical trial data and regulatory documents to patient education materials—while maintaining accuracy and consistency. Managing content as discrete elements allows teams to adapt materials for multiple audiences and formats more efficiently, reducing both time and cost.
Unstructured content, by contrast, lacks a predefined data model. It is often text-heavy or multimedia-rich but difficult to repurpose. Traditional WYSIWYG interfaces make editing easy for non-technical users, yet they create challenges in scalability and quality control as websites and regulatory submissions become more complex.
Treating structured information as a governed business asset strengthens compliance and efficiency across geographies and platforms. Many life sciences organizations are now adopting structured approaches to define, model, and govern their content as part of broader digital transformation strategies.
Content Strategy in the Pharmaceutical Sector
A successful shift to structured content begins with a clear strategy: defining what information to create, how it will be organized, and how it will flow through authoring, review, and publishing. In pharma, content strategy must align with the industry’s unique priorities—regulatory compliance, data integrity, and patient safety. A strong governance framework ensures that every asset, from a product label to a regulatory submission, is consistent, reliable, and auditable. This alignment not only improves quality and communication but also positions organizations to scale as regulatory expectations evolve.
Why Architecture Matters Now
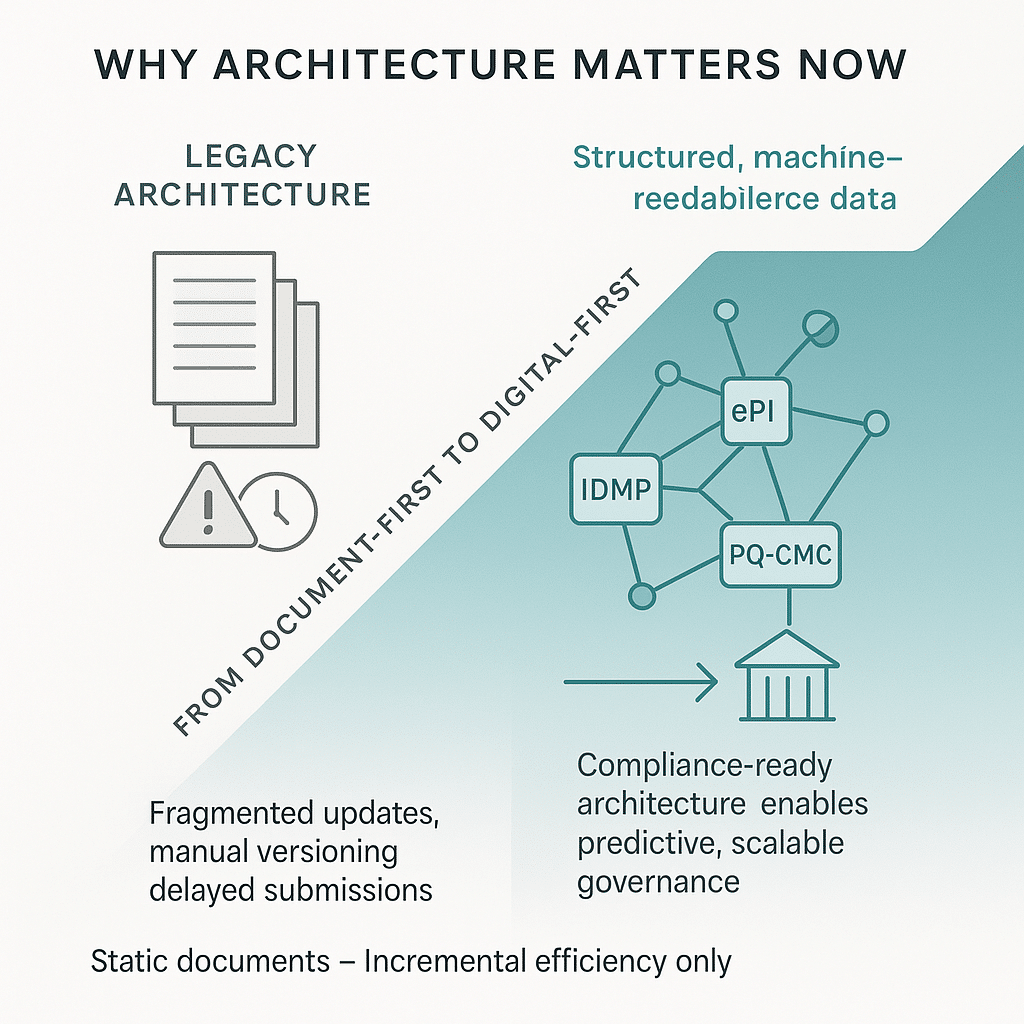
Content management systems designed for static documents once offered incremental efficiency. But regulators now require digital-first, machine-readable formats under standards such as ePI, IDMP, and PQ-CMC. Health authorities no longer accept static files—they expect structured, interoperable, compliance-ready data that can flow across systems.
Meeting these demands requires robust content modeling: defining content types, attributes, and relationships to form a scalable, governed environment. Without this foundation, organizations risk fragmented updates, versioning problems, and submission delays. Modern architecture turns compliance from a reactive function into a predictive capability, enabling proactive alignment with regulatory change.
From Document Management to Governance-First Ecosystems
Historically, content management focused on archiving and routing static documents such as clinical protocols, SOPs, and labeling artifacts. These tools ensured recordkeeping but rarely supported metadata, reuse, or automated compliance.
Today, regulators require information that is structured, interoperable, and component-based. This shift demands ecosystems that reuse content across functions and markets, automate auditability at the component level, and generate multiple outputs—such as FHIR XML, SPL, or JSON—from a single source. Effective systems integrate seamlessly with regulatory and analytics platforms, enabling consistency and efficiency across global submissions.
In these ecosystems, structured content functions as modular building blocks—paragraphs, data tables, figures, and labels—that can be updated once and automatically propagated wherever used. This ensures uniformity and dramatically reduces manual effort. When implemented well, such systems are metadata-rich, accessible to non-technical users, and optimized for both regulatory and digital publishing.
Five Content Architectural Approaches in Regulated Pharma Content
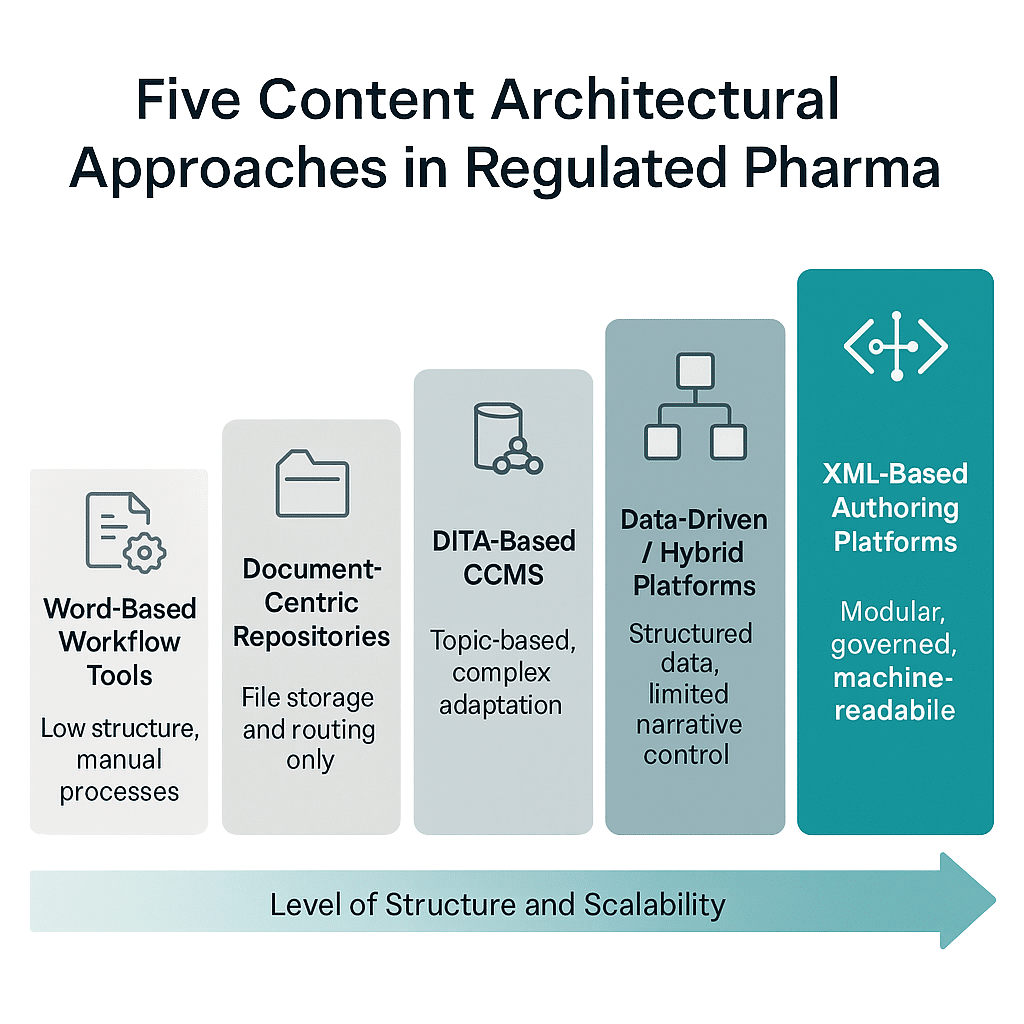
Not all platforms support structured content equally. Five architectural lineages dominate the field, each with distinct strengths and limitations:
| Word-Based Workflow Tools | Extensions of Microsoft Word that reduce friction but remain document-centric. Collaboration and scalability are limited, and they cannot produce regulatory-compliant, machine-readable outputs at enterprise scale. |
XML-Based Authoring Platforms | The backbone of scalable structured content. These treat information as metadata-rich components governed by a content model. XML outputs are inherently machine-readable, enabling automation and reuse. Many now support JSON, bridging modern APIs and regulatory systems. For pharma, this architecture (JSON) remains the most future-ready. |
Data-Driven or Hybrid Platforms | These generate documents directly from datasets and perform well in CMC contexts. However, they often lack narrative flexibility, multilingual capabilities, and enterprise governance. |
Document-Centric Repositories | Legacy repositories optimized for file storage and routing. They support recordkeeping but not structured metadata, reuse, or automation. Retrofitting them for modern requirements often leads to fragile, unsustainable solutions. |
DITA-Based CCMS | Originally built for software documentation. While topic-based authoring resembles structured principles, DITA’s adaptation to pharma is complex. Audit standards, compliance templates, and global labeling management often exceed its design scope. |
Structured Labeling: Managing Global Complexity
Labeling reveals why this transition is urgent. Regulators now expect dozens of synchronized local variants with traceability to source components. Effective architectures must support market-specific metadata, traceability from component to submission, and multilingual version control. AI-assisted impact analysis can accelerate updates, while XML/JSON compatibility ensures alignment with authority systems. Without this foundation, teams face manual retrofits and repeated validations; with it, global consistency becomes achievable.
The Emergence of AI
Artificial intelligence is now embedded in modern platforms. AI tools extract metadata, flag inconsistencies, automate compliance verification, and conduct change-impact analyses. Yet AI’s reliability depends entirely on architecture. Only machine-readable, metadata-rich content can support trustworthy, compliant automation. Poorly structured systems introduce risk rather than efficiency.
When applied to governed content, AI reduces submission delays, minimizes manual errors, and strengthens regulatory assurance. In practice, this means faster compliance checks and more confident decision-making.
Accessibility and Digital Reach
Accessibility is both a regulatory and ethical requirement. Structured content—with clear headings, lists, tables, and metadata—makes information predictable and user-friendly across devices and assistive technologies. It also supports discoverability by improving metadata consistency and search optimization. The result is information that is both compliant and accessible to those who depend on it.
Enabling Transformation: People, Process, Technology
Adopting structured systems is not only a technical upgrade—it’s an organizational transformation. Success depends on people, process, and technology working together. Teams must learn new skills in metadata management and governed authoring. Governance frameworks and automated workflows ensure consistency, while technology integrates with regulatory portals, analytics, and SEO tools. When aligned, these dimensions enable scalable, compliant information ecosystems across geographies.
Measuring ROI
The impact of structured content can be measured through clear metrics: reuse rates across submissions, reductions in creation and update times, improved SEO performance, and stronger compliance outcomes. Tracking these KPIs allows organizations to quantify ROI—showing tangible improvements in efficiency, risk reduction, and content visibility.
Overcoming Challenges
Transitioning to structured approaches involves challenges: upfront investment, skill development, and cultural adaptation. The solution is a phased rollout supported by pilot projects and continuous training. When executed thoughtfully, structured content reduces repetitive work, accelerates timelines, and improves compliance quality.
Key Evaluation Questions for Leaders
When evaluating a platform, leaders should ask:
Does it enforce reuse at the component level?
Are governance, audit trails, and traceability embedded?
Does it support XML, JSON, SPL, and regulatory integration?
Was it designed for pharma use cases?
Can it scale across 50+ markets and multiple regulated content types?
Does it embed AI in governed, auditable workflows?
Will it remain compliant as regulatory standards evolve?
These questions separate scalable, future-proof investments from temporary solutions.
Structured Architecture as Competitive Advantage
Ultimately, structured content is an architectural commitment that defines how teams collaborate and how organizations adapt to regulatory change. Companies relying on static document workflows remain locked in manual cycles of revision and revalidation. Those adopting pharma-native, interoperable architectures gain the ability to govern, comply, and accelerate globally. As AI becomes intrinsic to regulatory intelligence, governed, interoperable architectures will determine which organizations lead the field.
This blog summarizes insights from our latest executive report. For a deeper dive, download the full white paper:
Download the report: Navigating the Structured Content Landscape in Life Sciences