The Case for AI in Pharma Structured Content
Traditional document-centric processes—reliant on static Word files and siloed repositories—are reaching their breaking point. Structured Content Authoring (SCA) has emerged as a proven strategy for addressing these challenges. When coupled with artificial intelligence (AI), it doesn’t just improve efficiency—it transforms how organizations create, manage, and govern regulatory content at scale.
This evolution is no longer hypothetical. Leading pharmaceutical companies are implementing AI-enhanced structured content models, building AI capabilities on top, and reaping measurable benefits in speed, accuracy, and compliance readiness. But success depends on more than technology adoption; it requires a shift in mindset, governance, and operational discipline.
The State of Content Modernization
1. Traditional CMS and Document Models Are Functionally Insufficient
Legacy content management systems and document-based workflows were designed for static authoring and linear review—not for modularity, dynamic reuse, or real-time version control. As a result:
- Content updates across global product families become manual and error-prone.
- Regional labeling teams struggle to maintain consistency and traceability.
- Regulatory deadlines are missed due to inefficient reconciliation efforts.
These systems lack the metadata architecture and automation required to support modern regulatory requirements. Continuing to rely on disconnected documents increases both operational cost and compliance risk.
2. AI-Enhanced Structured Content Is Not a Formatting Strategy—It Is a Governance Model
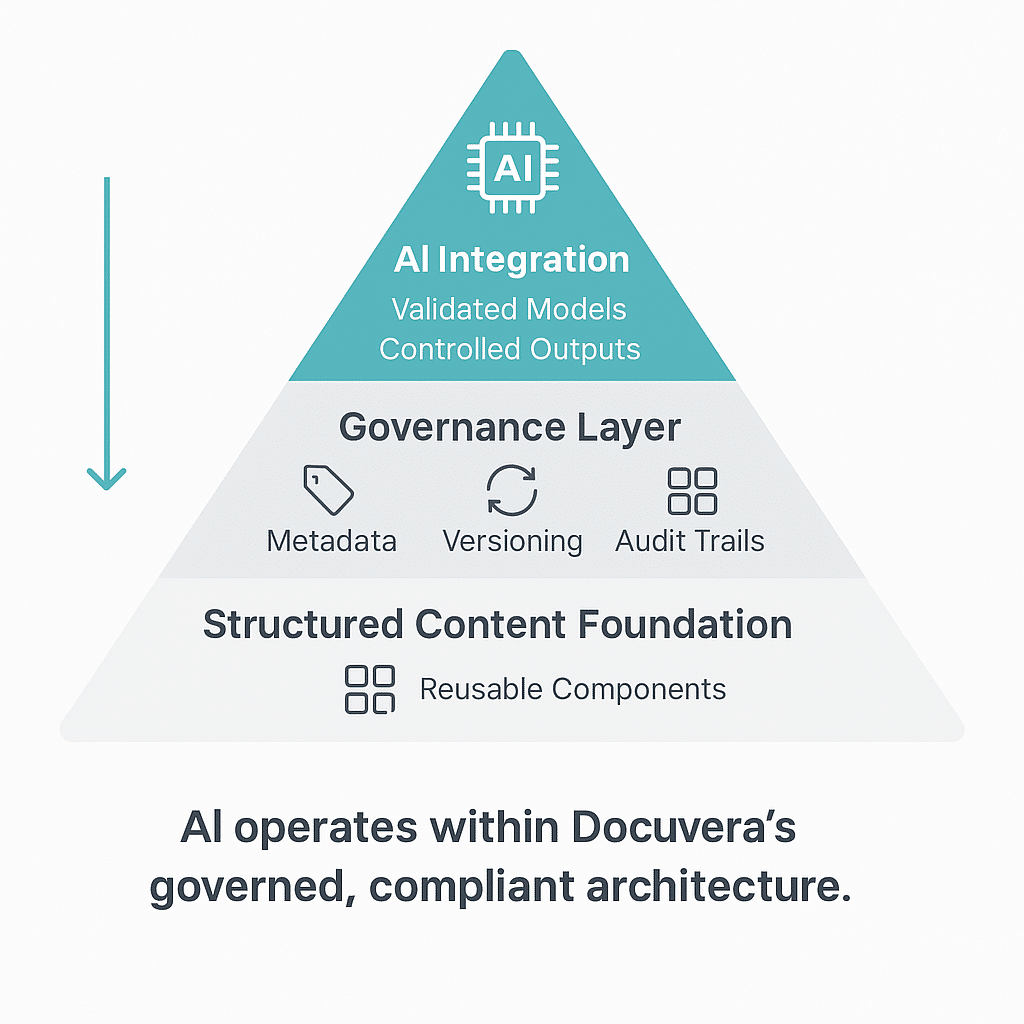
Structured content goes beyond converting documents into XML or applying templates. It is an enterprise-wide approach to content governance. Key components include:
- Content modularization: Breaking documents into reusable components at the sentence or paragraph level.
- Metadata tagging: Applying controlled vocabularies and taxonomies for traceability and automation.
- Reuse enforcement: Implementing processes and systems that ensure components stay synchronized across markets and documents.
When executed well, this model eliminates duplication, strengthens compliance, and accelerates change control—particularly in labeling, where regional variations and frequent updates introduce significant complexity.
3. AI Adds Measurable Value—But Only on a Structured Foundation
AI is often viewed as the silver bullet for content problems, but without structured data, it is an empty promise. Why? Because AI models require standardized, modular, and metadata-rich content to function effectively.
- In a structured environment, AI can automate translations, predict regulatory changes, and assist with content validation.
- Without structure, AI becomes a patchwork solution that amplifies inconsistencies rather than resolving them.
Think of AI as an acceleration layer—not the core capability. The real transformation starts with structured content.
The Organizational Shift Is Strategic
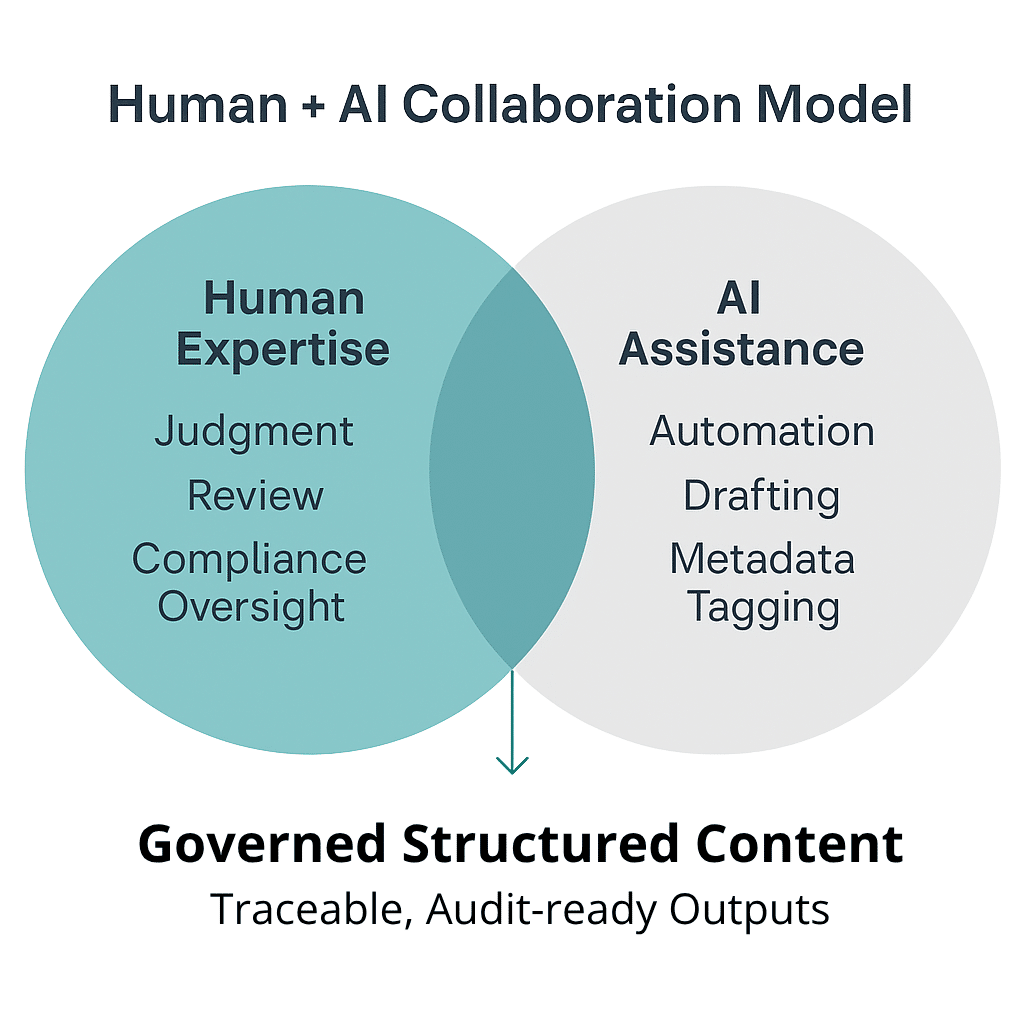
Transitioning to AI-powered structured content is not just an IT project. It is an enterprise transformation that impacts how:
- Regulatory and labeling teams author, assemble, and approve content
- Cross-functional stakeholders manage version control at the component level
- Organizations govern compliance across global submissions
It also requires a culture shift:
- From linear authoring → parallel content assembly
- From file management → reuse governance
- From individual ownership → cross-functional stewardship
Leadership alignment and clear governance frameworks are non-negotiable for success.
Future Regulatory Landscape: Why This Matters Now
Health authorities worldwide are moving toward machine-readable, structured submissions that demand component-level traceability. Regulatory initiatives such as ePI (electronic Product Information), IDMP (Identification of Medicinal Products), and CTD 4.0 will require companies to demonstrate content lineage across markets, updates, and lifecycle events. Organizations that fail to modernize risk not just inefficiency, but non-compliance.
How to Get Started
- Conduct a Content Audit: Identify duplication, inconsistencies, and high-impact labeling processes.
- Define a Metadata Strategy: Establish controlled vocabularies to support automation and traceability.
- Select a Pilot Use Case: Start with a single, high-value labeling workflow.
- Validate Structure Before AI: Ensure modular content is stable before introducing automation.
- Measure Everything: Track reuse, cycle time, and compliance metrics to quantify ROI.
Why Docuvera Is Built for This Shift
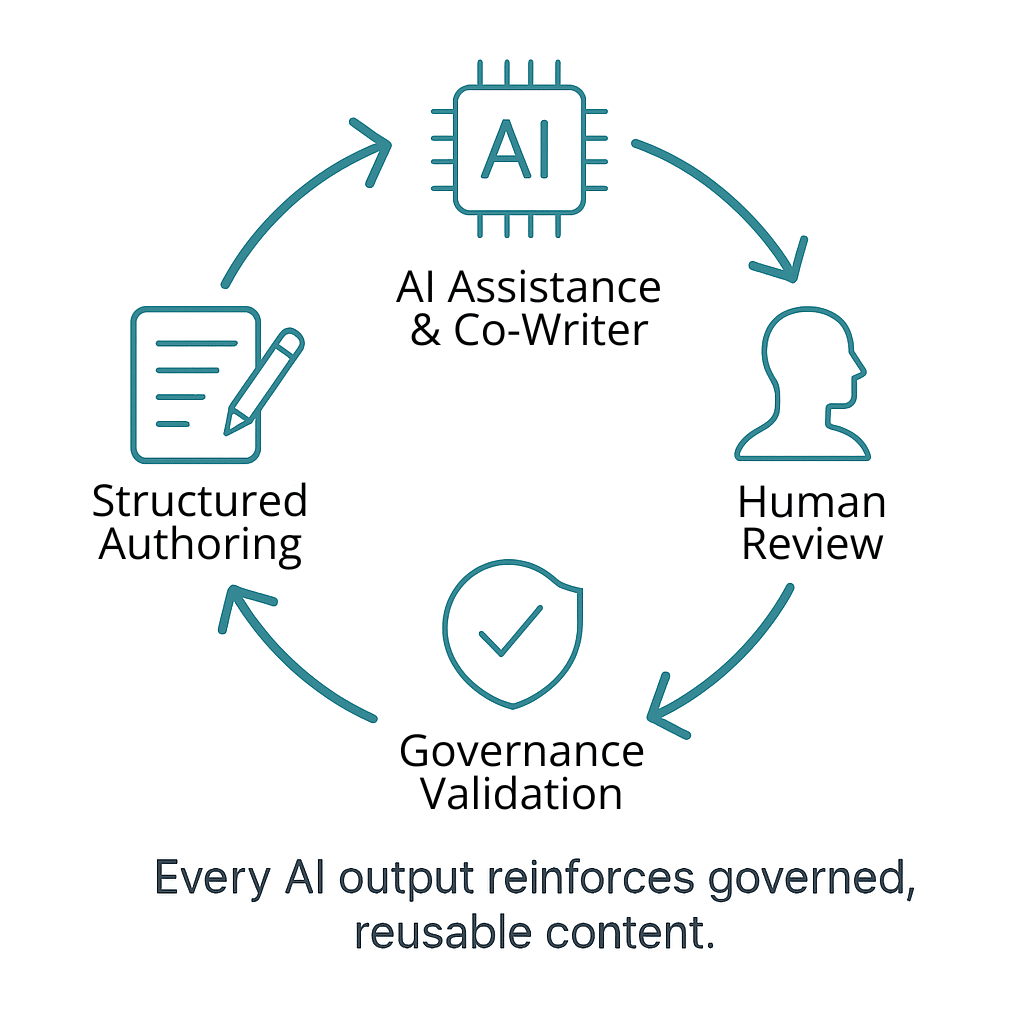
Docuvera was designed from the ground up to help life sciences companies move beyond document-based processes and into a structured, AI-ready ecosystem. Our platform enables:
- True modular content authoring: Breaking regulatory content into reusable, version-controlled components.
- Metadata-driven governance: Ensuring compliance, traceability, and automation readiness across global markets.
- AI integration that matters: From intelligent content assembly to compliance validation, our AI capabilities build on a structured foundation—not the other way around.
Unlike traditional systems that retrofit modularity into legacy workflows, Docuvera starts with structured content as the core design principle, making it the most effective way to prepare for evolving regulations and leverage AI for real business value.
If your organization is ready to modernize labeling and regulatory content management, we’re ready to help you get there.
Executive Perspective: Why This Is a Leadership Imperative
The path to AI-enhanced structured content is no longer a competitive advantage—it’s a compliance necessity. Regulatory expectations are moving faster than traditional processes can accommodate. Organizations that wait risk falling behind not just on speed and cost, but on market access and regulatory trust.
Technology is critical, but leadership alignment is the real differentiator. Companies that invest in governance, process maturity, and the right platforms now will set the standard for the next decade of regulatory excellence.
The question is no longer whether to move to structured content. It’s whether you’ll do it before your competitors—and before regulators force your hand.