Aligning Structured Content with Global Regulatory Mandates and Enterprise Transformation
The pharmaceutical industry today faces an unprecedented convergence of rising regulatory demands, expanding global markets, and the accelerating push toward digital-first operations. Traditional document-centric approaches—static documents, manual updates, and siloed systems—can no longer keep up with the constantly evolving regulatory standards and the complexity of the regulatory process. To ensure compliance and maintain competitive advantage, enterprises need a new foundation: AI-powered Structured Content Authoring (AI-SCA).
Clarifying the Landscape:
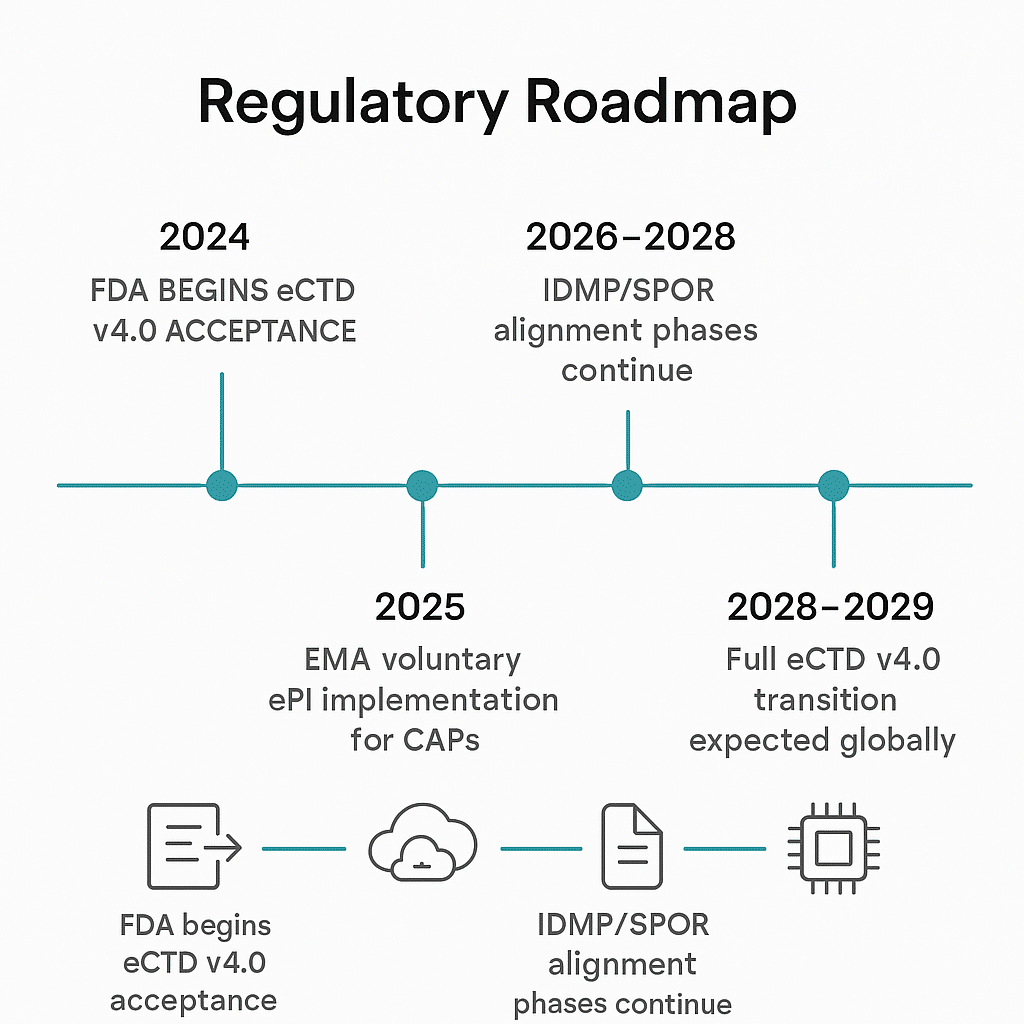
The Common Technical Document (CTD) defines how content is organized for global submissions, while the electronic Common Technical Document (eCTD 4.0) defines how that content is electronically submitted using XML backbones, metadata, and lifecycle operations. This next-generation standard—mandated by FDA and EMA over the coming years—marks a fundamental shift toward structured, interoperable regulatory data.
This transformation is not just a technology upgrade; it represents a fundamental mindset shift toward modular, intelligent, and reusable content that supports regulatory submissions, accelerates approval processes, and enhances drug safety documentation.
Why Now? The Regulatory Landscape Is Changing
The regulatory environment, especially in the pharmaceutical industry, is more complex and dynamic than ever before. Regulatory agencies such as the FDA (Food and Drug Administration) in the United States government and the European Medicines Agency (EMA) are mandating new data formats, standardizing data exchange protocols, and imposing tighter timelines for submissions and approvals.
Key shifts reshaping the landscape include:
- Global complexity: More markets, more regional variations, and diverse submission formats require companies to structure content that can be adapted for multiple regulatory jurisdictions.
- Digital mandates: Initiatives like electronic Product Information (ePI), Identification of Medicinal Products (IDMP), and the emerging electronic Common Technical Document (eCTD) 4.0 demand structured, machine-readable content that supports faster and more accurate review.
- Operational bottlenecks: Traditional document-driven workflows are slow, fragmented, and prone to errors, increasing risk and delaying the regulatory approval process.
Industry analyses show that 40–60 % of regulatory submission delays stem from manual document compilation and inconsistent source data. Structured content reuse can reduce authoring time by up to 50 %, freeing resources for strategic review.
Pharmaceutical companies and drug manufacturers that cling to manual processes risk falling behind in regulatory compliance and market access. Those that adopt structured, AI-enabled approaches gain the ability to ensure drug safety, improve efficiency in manufacturing operations, and navigate the regulatory process with greater agility.
What Is Structured Content Authoring (SCA)?
Structured Content Authoring (SCA) is a method of creating content by breaking down documents into modular, reusable components or content blocks. Each block carries metadata—machine-readable tags that define critical elements such as product name, indication, dosage form, or geographic region.
This approach enables pharmaceutical companies to create content once and reuse it consistently across various contexts, including regulatory submissions, clinical trials documentation, safety updates, and patient communications. By structuring content, companies ensure consistency, accuracy, and traceability across all documentation, which is essential for regulatory compliance and quality assurance.
What Makes It AI-Powered?
- Metadata Tagging: AI automatically classifies components, reducing manual effort and ensuring alignment with eCTD 4.0 metadata vocabularies.
- Smart Reuse: Intelligent search surfaces content already approved or referenced elsewhere, minimizing re-authoring.
- Inconsistency Detection: AI identifies conflicting data or outdated values across reused components.
- Predictive QA: Suggests corrections and completeness checks prior to publishing.
This intelligent automation enhances the ability of regulatory teams to manage complex documentation efficiently while maintaining rigorous standards for drug safety and quality.
Strategic Alignment with Global Digital Initiatives
AI-SCA directly supports the regulatory mandates and digital initiatives shaping the pharmaceutical industry’s future:
- ePI: Structured content is essential for future electronic Product Information formats, including FHIR-based XML. AI-SCA enables rapid assembly, validation, and updating of product labeling and safety information.
- IDMP: By tagging content with standardized identifiers (substance, product, dose, route), AI assists in auto-tagging and validation, helping companies comply with EMA’s SPOR data requirements and other global regulations.
- eCTD 4.0: As regulators move toward structured data submissions, AI-SCA allows pharmaceutical companies to modularize and align content with evolving templates and data exchange standards, streamlining the approval process.
Benefits Across the Regulatory Lifecycle
Implementing AI-SCA delivers significant benefits throughout the regulatory lifecycle:
- Speed: Accelerates content creation, review, and approvals, helping companies meet tight regulatory deadlines.
- Scale: Enables high reuse of content across global markets, product lines, and submission types, reducing duplication and effort.
- Quality: Predictive validation and inconsistency detection lower the QA burden and reduce the risk of regulatory non-compliance.
- Readiness: Keeps enterprises prepared for new mandates like IDMP, ePI, and CTD modernization, supporting continuous compliance with regulatory standards.
Integration with the Regulatory Tech Stack
AI-SCA complements and strengthens existing regulatory technology systems:
- Regulatory Information Management (RIM): Links structured content to authoritative product data and submission status tracking.
- Document Management Systems (DMS): Supports existing workflows for review, approval, and archival, while improving consistency and traceability.
- Submission tools and portals: Provides validated, structured content ready for regulatory delivery, ensuring compliance with agency requirements.
By serving as the connective tissue within the regulatory tech stack, AI-SCA enables seamless collaboration and efficient regulatory operations.
From Document-Driven to Data-Driven: The Maturity Model
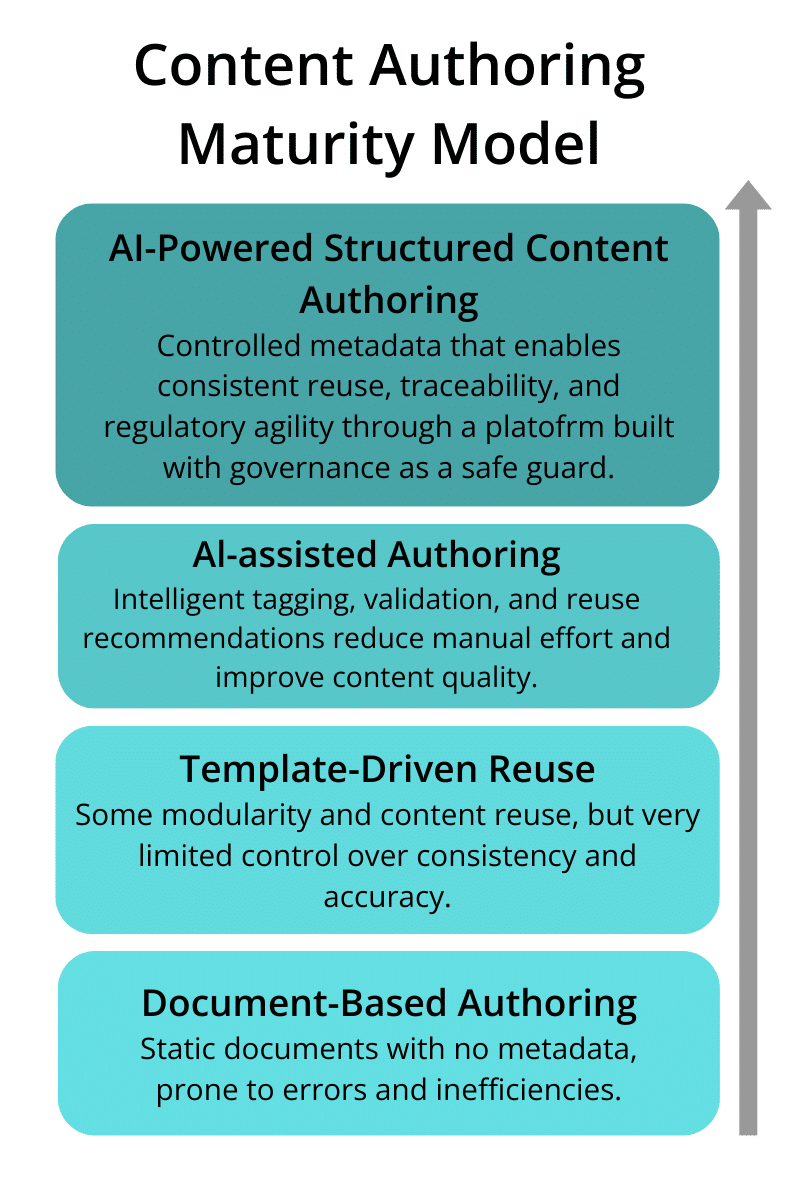
Every organization sits somewhere on the journey from static documents to dynamic data.
Level 1: Document-centric, manual assembly
Level 2: Reuse awareness, partial templates
Level 3: Component-based authoring in select functions
Level 4: Enterprise-wide metadata and AI-assisted reuse
Level 5: Fully data-driven submissions aligned with eCTD 4.0 and HL7 FHIR
Understanding your current level helps define where to focus first—whether it’s labeling, CMC, or clinical summaries.
Getting Started: A Phased Roadmap
Transformation to AI-SCA is a strategic journey that requires deliberate planning:
- Identify 1–2 high-value use cases that will demonstrate impact.
- Audit current content and metadata readiness to understand gaps.
- Select an AI-SCA platform aligned with regulatory needs and integration capabilities.
- Launch a pilot with measurable KPIs focused on speed, quality, and compliance improvements.
- Expand adoption through governance, training, and integration across RIM, DMS, and submission systems.
Key Questions for Strategic Alignment
For Regulatory Leaders:
- Map AI-SCA adoption to eCTD 4.0 timelines and IDMP compliance programs.
- Establish governance roles early to ensure metadata consistency.
For Authors & Publishers:
- Focus pilots on repetitive documents (labels, SmPC, IB).
- Capture feedback loops between AI suggestions and SME review cycles.
Where Docuvera Fits In
Docuvera empowers pharmaceutical companies and drug manufacturers to realize the benefits of AI-SCA:
- Structured from the ground up: Content modularization, metadata governance, and AI-powered authoring designed specifically for regulatory complexity.
- Aligned to regulatory mandates: Supports ePI, IDMP, eCTD 4.0, and other evolving requirements from agencies like the FDA and EMA.
- Ecosystem-friendly: Seamless integration with RIM, DMS, and submission tools to ensure regulatory compliance and operational efficiency.
- Enterprise-ready: Scalable across functions, products, and global markets, supporting business growth and regulatory agility.
With Docuvera, structured content becomes a practical capability that enhances drug safety documentation, accelerates the approval process, and ensures compliance with rigorous regulatory standards.
The Bottom Line
Structured content is no longer optional for pharmaceutical companies aiming to thrive in a constantly evolving regulatory landscape. AI-SCA is becoming the operational foundation for regulatory agility, compliance, and enterprise integration.
The path forward is clear:
- Begin with structured content and metadata governance.
- Layer in AI-driven intelligence to improve accuracy and efficiency.
- Build for speed, scale, and auditability to meet regulatory challenges head-on.
Docuvera is ready to help organizations accelerate this transformation. The future of regulatory operations is structured, intelligent, and integrated—and it’s already within reach.