For years, pharmaceutical companies have built their regulatory operations around traditional documents. Teams created, edited, and shared Word files, PDFs, and spreadsheets in workflows that were sufficient when submission volumes were smaller and regulatory requirements relatively stable.
However, those days are gone. Today, global health authorities demand not only speed but also consistency, accuracy, and digital-ready formats that enable content reuse across regions and products. At the same time, pharmaceutical companies are managing increasingly complex product pipelines, expanding into more markets, and facing greater variation in how content must be presented and approved.
This post is Part 1 of a two-part series exploring how artificial intelligence and structured content intersect to transform regulated life sciences documentation.
Traditional document-centric workflows are buckling under this growing pressure. What was once a manageable administrative task has now become a strategic risk, leading to submission delays, increased scrutiny from health authorities, lost revenue from slower product launches, and reputational damage due to non-compliance. Structured authoring helps maintain compliance with regulatory requirements by ensuring content is accurate, consistent, and meets strict industry standards.
This is why structured content authoring (SCA) has transitioned from being a “nice-to-have” efficiency tool to becoming the backbone of regulatory transformation in the pharmaceutical industry. Organizing content into modular, reusable components is a strategic approach that enhances efficiency, consistency, and standardization across documents. A good example of structured content is a resume or a recipe, where information is clearly organized into sections for easy readability and reuse. By breaking content into modular, reusable components, SCA enables teams to maintain a consistent format and ensures content reuse across multiple documents and regulatory submissions. When enhanced with artificial intelligence (AI), structured authoring not only accelerates workflows but also improves accuracy and compliance, making regulatory processes smarter and more reliable.
Why Now? The Pressures Shaping Regulatory Operations
Expanding Global Reach and Diverse Formats
Pharmaceutical companies no longer submit regulatory documents to just a handful of major markets. Each new product launch involves dozens of regional variations, each with unique rules, languages, and templates, and the need to distribute content across multiple channels and various platforms. Without a structured authoring system to trace and reuse approved content efficiently, regulatory teams are forced into repetitive, error-prone manual work, increasing the risk of inconsistencies and compliance issues.
Evolving Regulatory Mandates and Digital Initiatives
The regulatory landscape is evolving rapidly, with initiatives such as:
- IDMP (Identification of Medicinal Products), which introduces structured data standards affecting how products, substances, and doses are recorded.
- CTD 4.0 (Common Technical Document version 4.0), pushing submissions toward modular, structured frameworks. Utilizing xml schema and a predefined content structure is essential for ensuring that content is consistently organized and interoperable, meeting new regulatory requirements.
- ePI (electronic Product Information) initiatives across Europe and beyond, signaling a digital-first future for product information management.
Traditional document-centric processes simply cannot keep pace with these complex and evolving requirements.
Accelerated Timelines and Cost Pressure
Blockbuster drugs are becoming rare, and competitive launches are the new norm. Every week shaved off a regulatory cycle can translate into millions of dollars in earlier revenue. Delays caused by redundant content reviews, inconsistent documentation, or manual reconciliation are no longer tolerable in this high-stakes environment. To meet accelerated timelines and maintain consistency, efficient workflows are essential for streamlining content creation and ensuring reliable processes.
What Is Structured Content Authoring (SCA)?
Structured Content Authoring (SCA) is a method of content creation that breaks down regulatory documents into modular, reusable content components.
Structured content is organized in a structured format using predefined schemas or templates, which makes it easier to manage, reuse, and maintain consistency at scale. In contrast, unstructured content is stored in formats like Word or Google Docs without a defined structure, making it more flexible but harder to organize, analyze, or update across entire documents. Many organizations use both structured and unstructured content depending on their communication needs, but structured content is preferred for large-scale or regulated documentation because it supports regulatory requirements and improves content quality.
Instead of producing static documents from scratch each time, content is authored as discrete blocks enriched with metadata—such as product information, market, indication, and version—that can be easily traced, tagged, and governed. A content model defines the structure and relationships of these modular components, supporting efficient content development and management.
- These content blocks can be dynamically assembled to form complex regulatory submissions like SmPCs (Summary of Product Characteristics), CCDSs (Company Core Data Sheets), CTDs, clinical trial protocols, or brochures.
- Updates made to a single content component automatically cascade across all related documents, ensuring consistent messaging and reducing the risk of discrepancies. This process ensures uniformity and helps maintain consistency and compliance.
Structured content authoring tools help organizations create, manage, and reuse content efficiently by enabling separating content from formatting, which allows content to be reused across other formats and multiple outputs such as PDFs, HTML, or help files.
The benefits of structured content authoring include content reusability, support for multiple outputs, and more efficient workflows. Reusable elements like snippets and variables streamline content development and updates, while a component content management system and content management system provide the technical framework for scalable, accurate, and consistent content management. Using the right tools enables writers to focus on content creation and produce content efficiently, rather than spending time on formatting or manual updates.
This approach enables regulatory teams to work from a single source of truth, streamlining the content creation process and improving both efficiency and compliance.
How AI Enhances Structured Content Authoring Tools
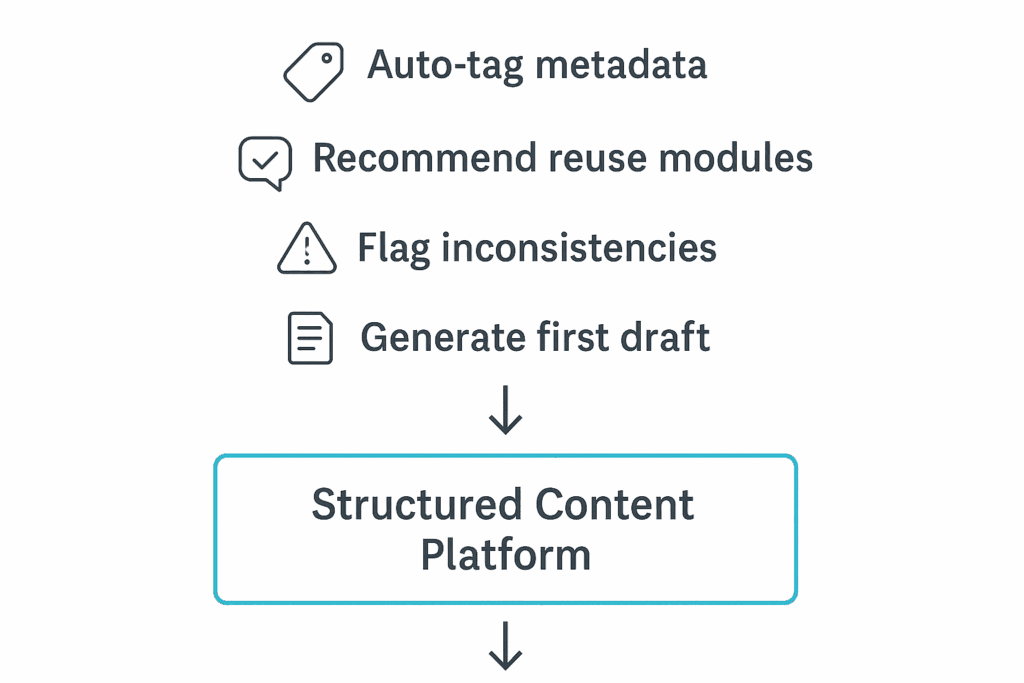
AI acts as a powerful accelerator on top of SCA by automating and optimizing many aspects of the authoring process:
- AI algorithms suggest the most appropriate content blocks to reuse based on product attributes and regional requirements.
- Natural Language Processing (NLP) auto-tags content with relevant metadata, reducing manual effort.
- AI flags inconsistencies and potential compliance risks across related submissions before they reach health authorities.
- It can generate first drafts for content-heavy sections such as safety narratives, enabling technical and medical writers to focus on refinement and review.
AI-powered structured content authoring also leads to improved collaboration among regulatory teams by streamlining teamwork and ensuring consistency across documents.
Far from replacing human expertise, AI amplifies it—reducing manual burdens while enhancing confidence in every submission. AI further contributes to enhancing efficiency and customer satisfaction by enabling faster, more accurate content delivery across multiple channels.
Structured Content Authoring and Collaboration
Structured authoring and collaboration are transforming the way technical documentation is created, managed, and maintained. By leveraging structured authoring tools, organizations can empower technical writers to focus on content creation while ensuring that content is consistently organized and easily reusable across multiple documents and outputs.
A key advantage of structured content authoring is the separation of content from formatting. Using markup languages such as XML, technical writers can develop content components that are independent of layout, making it simple to update, reuse, and repurpose information for different formats and regulatory requirements. This structured approach not only streamlines the writing process but also supports compliance by maintaining a consistent format and messaging throughout all technical documentation.
Structured content authoring tools also improve content quality and accessibility. By organizing information into reusable components and applying consistent content rules, technical writers can ensure that documentation is clear, accurate, and easy to navigate. The inclusion of caption and alternative text supports accessibility, making content usable for all audiences, including those with disabilities.
Alignment with the Digital Regulatory Roadmap for Regulatory Compliance
The shift toward structured and intelligent content is inevitable and essential for future regulatory success:
- ePI initiatives require product information to be managed in machine-readable, structured formats.
- IDMP compliance will soon be mandatory, standardizing how product data is recorded and shared.
- CTD 4.0 envisions modular submissions that can only be enabled through structured content authoring and automated workflows.
Regulatory compliance is increasingly critical, and structured authoring helps organizations maintain compliance by ensuring content accuracy, supporting adherence to evolving regulatory standards, and streamlining updates as requirements change.
Organizations that delay modernization risk falling behind, while those that adopt AI-powered structured authoring platforms will benefit from smoother transitions, faster approvals, and scalable global operations.
The Real Cost of Maintaining the Status Quo
It may seem easier to continue managing regulatory content with traditional documents, but the hidden costs are significant. Without modern systems to manage content, organizations struggle to organize, assemble, and control information efficiently, leading to increased errors and inefficiencies:
- A single health authority query can delay submissions by months.
- Manual reconciliation of content across 20+ markets drains time and morale.
- Compliance gaps risk reputational damage and loss of trust with regulators.
Investing in structured content authoring tools and modern content management systems is no longer optional—it is critical to mitigating these risks and enabling regulatory agility.
Where To Go From Here
The pharmaceutical industry stands at a pivotal crossroads. One path leads to continued struggles with fragmented Word and PDF files, hoping manual oversight will catch every error. The other embraces AI-powered structured content authoring tools that lay the foundation for regulatory agility, accuracy, and speed.
In part two of this series, we will explore how to implement structured authoring effectively, what features to look for in an AI-SCA platform, common pitfalls to avoid, and how to make a confident, future-proof investment in your regulatory content processes.