In part one of this series, we explored the mounting pressures faced by modern regulatory teams—rising submission volumes, evolving standards like IDMP, and digital initiatives such as electronic Product Information (ePI)—that are rendering traditional document workflows unsustainable. The solution lies in adopting AI-structured content authoring (AI-SCA) platforms, which combine the power of artificial intelligence with the efficiency and compliance benefits of structured authoring. Organizing content is a core aspect of structured authoring, enabling a systematic, rule-based approach to managing large volumes of complex and regulated information.
However, recognizing the need for AI-structured content is only the first step. The critical question remains: which platform should your organization choose? The right tools can transform your regulatory content creation processes, enhance compliance, and improve user adoption. Conversely, the wrong choice could lead to siloed content, increased compliance risks, and frustrated users.
This guide will help pharma leaders navigate the vendor selection process with confidence and highlight how platforms like Docuvera are engineered to meet the complex demands of pharmaceutical regulatory operations. By leveraging structured authoring, documentation processes can be streamlined and made more efficient. The guide will also show how organizations create and maintain technical documentation through structured authoring, and how you can implement structured authoring to improve collaboration, ensure consistency, and meet compliance requirements.
Recap on Structured Content Authoring
Structured content authoring is a systematic approach to creating and managing content using a standardized, structured format. Unlike traditional methods, structured authoring tools empower technical writing teams to build technical documentation that is consistent, accurate, and easily scalable across multiple documents and channels. By leveraging structured content authoring, organizations can break content into reusable components, ensuring that each piece of information is maintained in a single location and can be updated universally. This separation of content from formatting not only streamlines the authoring process but also helps maintain consistency and compliance—critical factors in regulated industries like pharmaceuticals. With structured authoring tools, teams can efficiently manage content components, reduce duplication, and ensure that technical documentation meets the highest standards for regulatory compliance and quality.
Benefits of AI-Structured Content Authoring
Adopting structured authoring tools brings a host of benefits to organizations focused on technical documentation. One of the primary advantages is the ability to manage content through a component content management system (CCMS), which allows for the efficient reuse of content components across multiple documents and outputs. This approach not only saves time but also ensures that updates are reflected consistently wherever the same content appears. Structured authoring tools support version control, making it easy to track changes and maintain a single source of truth for all documentation. Additionally, organizations can produce content in various formats—such as XML, HTML, and PDF—enabling seamless publishing across different platforms and channels. The benefits of structured authoring extend to improved content quality, streamlined content management, and the ability to reuse content, all of which contribute to reduced production costs and enhanced compliance.
What Pharma Leaders Are Really Worried About
Before diving into technical features, it’s essential to understand the core concerns driving every buying decision:
- Regulatory Compliance: Will the platform meet global regulatory requirements such as IDMP, CTD, and ePI? Compliance is non-negotiable. The system must offer audit-ready traceability and support regulatory standards to maintain compliance.
- User Adoption: Will medical writers and regulatory teams embrace the new platform? The success of any AI-SCA tool hinges on user-friendly interfaces and efficient workflows that minimize resistance and maximize productivity. A well-defined content creation process is crucial for ensuring that medical writers and regulatory teams can efficiently create, reuse, and manage content.
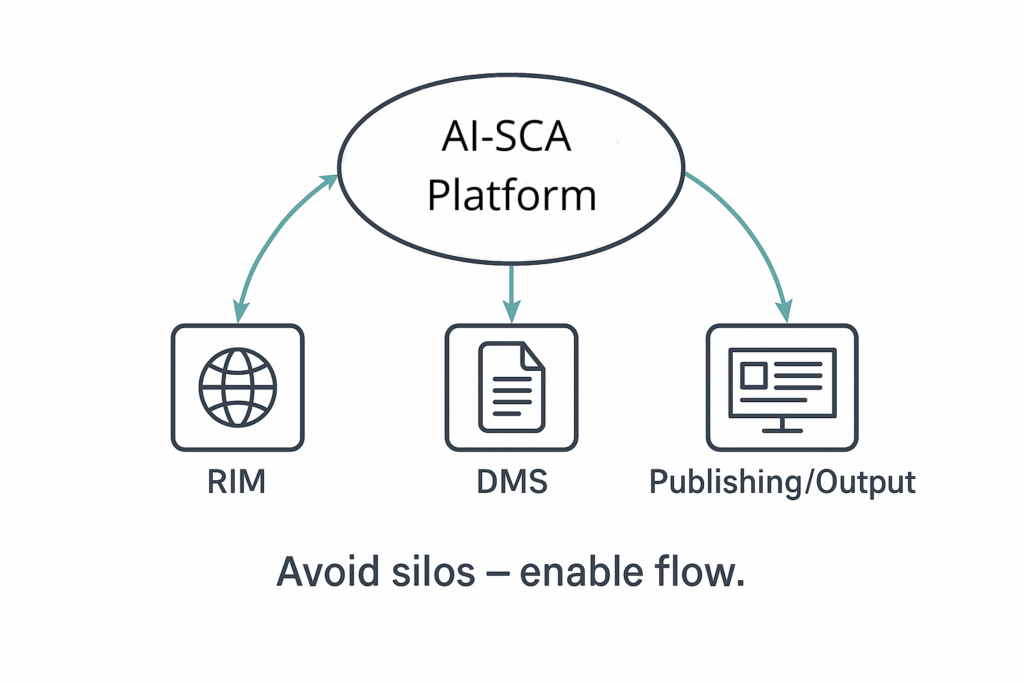
- Content Silos: Does the platform integrate seamlessly with existing systems like Regulatory Information Management (RIM) and Document Management Systems (DMS)? Avoiding fragmented content silos is critical for streamlined content management and reuse.
- Change Management: How will your organization manage the transition? The technical writing team plays a key role in managing the transition to new platforms, ensuring effective governance, training, and content migration strategies, which are as vital as the technology itself for successful implementation.
Choosing an AI-SCA platform should alleviate these concerns, not exacerbate them. Improved collaboration is a key benefit of selecting the right AI-SCA platform, as it streamlines teamwork and ensures consistency across all documentation.
What Makes a Platform Truly AI-Enabled?
Beware of vendors who overuse the term “AI” without delivering substantive capabilities. A credible AI-SCA platform should provide features aimed at enhancing efficiency through AI-driven automation and intelligent content management:
- Natural Language Processing (NLP): To automatically tag content with accurate metadata, enhancing content discoverability and reuse.
- Reuse Recommendations: AI-driven suggestions for content reuse based on regulatory history and context to accelerate the authoring process, maintain consistency, and support content reusability across multiple documents and channels.
- Inconsistency Detection: Identification of discrepancies across CTD modules or regional document adaptations, ensuring content quality and regulatory compliance.
- Transparent Audit Trails: Complete visibility into AI suggestions and changes, essential for regulatory audits and maintaining compliance.
Features That Separate Leaders from Pretenders
Modular Content Architecture for Structured Content Authoring
A true AI-SCA platform must separate content from formatting, enabling modular content architecture by structuring content within a modular framework. This ensures content components are reusable and not locked into templated documents disguised as “structured.” Organizing content systematically is essential to enable modularity, and a predefined content structure—such as using XML—supports efficient modular content creation. Look for platforms that support genuine structured authoring systems with a clear content model and component content management.
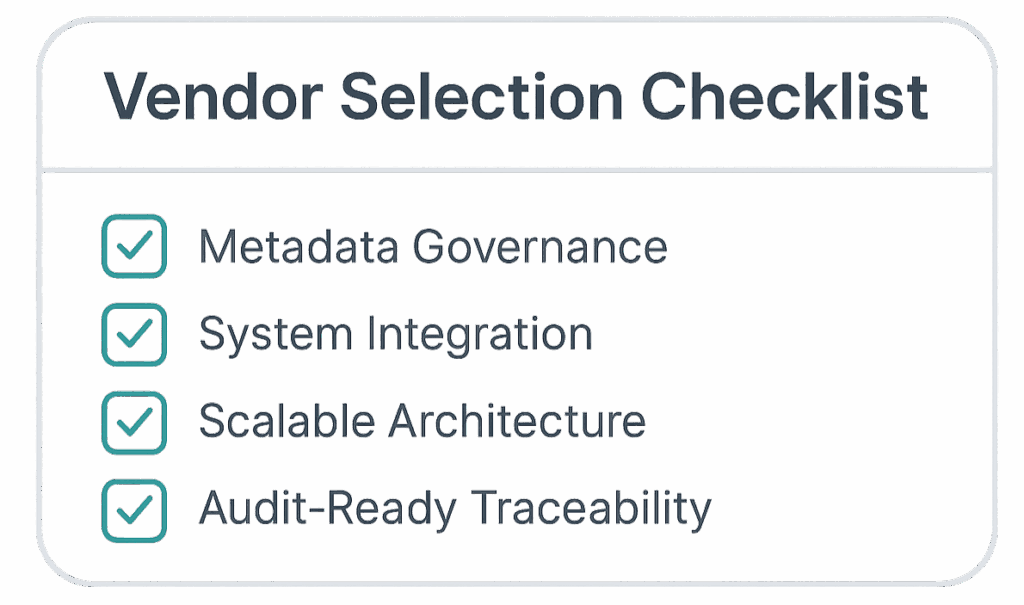
Metadata Governance
Metadata is the backbone of effective content reuse and compliance management. Leading platforms, such as Docuvera, enforce global taxonomies and ensure auto-tagging accuracy to maintain consistency across regions and markets.
Regulatory Alignment and Regulatory Compliance
Support for IDMP data models, readiness for CTD 4.0 modular submissions, and flexibility to publish to ePI initiatives are must-have features. The platform should align with evolving regulatory standards to future-proof your operations.
Seamless Integration
AI-SCA tools must integrate natively with your existing RIM and DMS systems to ensure smooth content flow and avoid creating new silos. Integration enhances content lifecycle management and version control.
Market and Language Awareness
Global pharma companies require AI-SCA platforms that can adapt content for multiple languages and regions, with AI suggestions that respect local regulatory rules and lexicons. This capability enhances localization efficiency and ensures consistent messaging worldwide.
Authoring Work and Collaboration
Effective authoring work and collaboration are at the heart of successful structured content authoring. Technical writers use structured authoring tools and markup languages like XML to organize and format content components, ensuring that information is both accurate and easy to manage. These authoring tools often feature real-time collaboration capabilities, such as commenting, version control, and task assignment, which enable multiple authors to work together efficiently on the same content. A user-friendly interface allows technical writers to focus on content creation rather than getting bogged down by formatting concerns. By separating content from formatting, structured authoring tools free writers to concentrate on producing high-quality, compliant documentation, while the system automatically applies consistent styling and structure across all outputs.
Common Buying Mistakes to Avoid
- Relying Solely on Demos: A visually appealing interface is insufficient if the platform cannot manage metadata effectively or enforce compliance.
- Confusing Templates with Structured Content: True structured authoring separates content from formatting and enforces content rules and governance, unlike templated documents. A common mistake is managing content as entire documents rather than as modular, reusable components.
- Equating AI with Full Automation: The real value of AI-SCA platforms lies in augmenting human expertise with smarter tools, not replacing human authors.
- Neglecting Change Management: Technology adoption depends heavily on governance, training, and user engagement strategies.
- Overlooking Multi-Format Publishing: Structured authoring tools enable multiple outputs, allowing you to publish content across multiple channels and in different formats, such as web, eBooks, CHM files, and other formats like Word, Google Docs, or XML. This versatility supports efficient content reuse and multi-channel publishing.
- Ignoring Future-Proofing: Consider platforms that support cloud based platforms for enhanced accessibility and collaboration, ensuring your content creation and management processes remain scalable and adaptable.
Common Challenges and Solutions
Implementing structured authoring can present several challenges, particularly when it comes to maintaining consistency across multiple documents and managing complex content. To address these issues, organizations can establish style guides, enforce content rules, and develop structured content models that standardize formatting and terminology. Structured authoring tools play a crucial role by enabling content reuse, automating publishing processes, and providing robust version control to track changes and updates. Managing complex content components—such as images, tables, and multimedia—is simplified through features that allow for easy integration and consistent application across technical documentation. By leveraging these solutions, organizations can ensure that their documentation remains accurate, consistent, and easy to maintain, even as content needs evolve.
Measuring Success and ROI
To justify the investment in structured authoring, organizations need to measure its success and return on investment (ROI) through clear, actionable metrics. Key indicators include improvements in content quality, reductions in production time, and lower maintenance costs for technical documentation. By comparing these metrics before and after implementing structured authoring, organizations can demonstrate tangible benefits. Additionally, analytics tools can track content usage, customer satisfaction, and other performance indicators, providing valuable insights into the effectiveness of documentation. Regularly reviewing these metrics allows organizations to refine their structured authoring processes, enhance content quality, and boost customer satisfaction—ultimately driving greater ROI and supporting long-term business success.
How Platforms Like Docuvera Help
Docuvera is purpose-built for life sciences, reflecting the realities of pharmaceutical regulatory complexity. Many organizations benefit from adopting structured authoring solutions like Docuvera to meet diverse communication needs. Organizations create and maintain technical documentation efficiently with Docuvera, ensuring consistency and compliance across teams and projects:
- Metadata-First Architecture: Ensures content is governed and reusable from day one, supporting efficient content creation, content reusability, and reuse of modular components.
- AI Transparency: Provides audit trails for every recommendation, supporting regulatory compliance and traceability.
- Out-of-the-Box Regulatory Alignment: Supports IDMP, CTD 4.0, and evolving ePI initiatives to keep your documentation aligned with current standards.
- Native Integrations: Seamlessly connects with RIM and DMS systems to prevent silos and improve operational efficiency.
- Scalable Adoption Pathways: Allows companies to start with high-impact use cases and expand as governance maturity grows, supporting content delivery across various platforms.
Conclusion
The case for adopting AI-powered structured content authoring in pharma is compelling: organizations need structured, intelligent content to meet modern regulatory demands and accelerate global product launches. However, the choice of platform will determine whether this modernization becomes a transformative leap or a costly misstep.
By focusing on features that truly matter—metadata governance, regulatory alignment, seamless integration, and AI transparency—pharma leaders can select a partner that ensures long-term agility, compliance, and content quality.
Platforms like Docuvera offer a future-ready solution, enabling pharma companies to confidently invest in their regulatory content processes and thrive in a complex, fast-changing environment.