The pharmaceutical industry operates within an increasingly complex regulatory landscape where accuracy, consistency, and compliance are non-negotiable. The FDA adopted the SPL standard to improve regulatory processes and ensure more efficient oversight. The FDA’s Structured Product Labeling (SPL) initiative represents a fundamental effort to transform traditional documentation into standardized, machine-readable formats, enhancing regulatory efficiency and patient safety for pharmaceuticals.
Standardization plays a crucial role in regulatory compliance, ensuring consistency and reliability across submissions. Historically, food safety regulations, such as the Pure Food and Drugs Act, influenced the development of modern drug labeling standards. Organizations leveraging structured content platforms like Docuvera demonstrate measurable advantages in meeting SPL requirements while accelerating time-to-market objectives. A recent article highlights the significant impact of the SPL initiative on the pharmaceutical industry.
Understanding FDA’s Structured Product Labeling Initiative
Core Framework and Technical Specifications
The FDA’s SPL initiative establishes a comprehensive framework for standardizing drug labeling information through structured, machine-readable formats. This system utilizes Health Level 7 (HL7) standards to create consistent data architecture across all pharmaceutical submissions. The FDA’s structured product labeling requirements mandate the use of electronic submissions in XML format. Creating SPL documents is a detailed and time-consuming process that requires careful preparation, review, and approval to ensure quality and compliance.
SPL technical requirements include:
- HL7 Version 3 Standards: Mandatory compliance with established messaging protocols
- XML Document Structure: Standardized markup language for consistent data formatting
- Controlled Vocabulary: Required use of FDA-approved terminology sets
- Digital Signatures: Electronic authentication mechanisms per 21 CFR Part 11, with a mechanism that ensures data integrity and document authenticity
- Lifecycle Management: Version control and change tracking capabilities
The data architecture also supports exchanging product and facility information efficiently using the SPL standard.
The initiative covers multiple document types essential to pharmaceutical operations. For further information or specific questions, please use our general inquiry form.
- Prescription Drug Labels
- Over-the-Counter (OTC) Monographs
- Establishment Registration Forms
- Drug Listing Information
- Labeling Supplement Documents
- Biologics, over-the-counter (OTC) products, and veterinary medicinal products
Regulatory Compliance Benefits
SPL implementation delivers quantifiable improvements in regulatory processes. The FDA reports a 67% reduction in labeling-related review cycles since SPL adoption began. Organizations using structured labeling demonstrate significantly fewer compliance deficiencies compared to traditional submission methods.
Notably, many organizations achieved significant milestones in the first year of SPL adoption, such as improved component reuse and streamlined submission workflows. Additionally, the FDA no longer accepts drug registrations and listings in paper forms, further emphasizing the importance of SPL compliance.
Key performance indicators include:
- Review Time Reduction: 45% faster FDA processing for SPL-compliant submissions
- Error Rate Improvement: 78% decrease in labeling inconsistencies
- Data Quality Enhancement: 92% improvement in information accuracy metrics
- Submission Efficiency: 56% reduction in resubmission requirements
- Faster Product Approval: Accelerated product approval timelines due to enhanced SPL compliance and efficient regulatory submissions
Impact on Life Sciences Teams
The pharmaceutical industry is experiencing a profound shift as life sciences teams embrace digital transformation and structured content to meet the demands of stronger compliance and improved consistency. In an environment where regulatory and clinical requirements are constantly evolving, these teams are leveraging advanced technologies to streamline processes, enhance quality, and drive operational efficiency. Structured content authoring in pharmaceutical documentation enables efficient content reuse and captures audit trails for compliance. By adopting structured content strategies, life sciences teams are not only reducing costs but also ensuring that their pharmaceutical products meet the highest standards of safety and efficacy. This transformation is enabling organizations to respond more rapidly to regulatory changes, maintain compliance across global markets, and deliver high quality content that supports both business objectives and patient outcomes.
Transforming Cross-Functional Collaboration
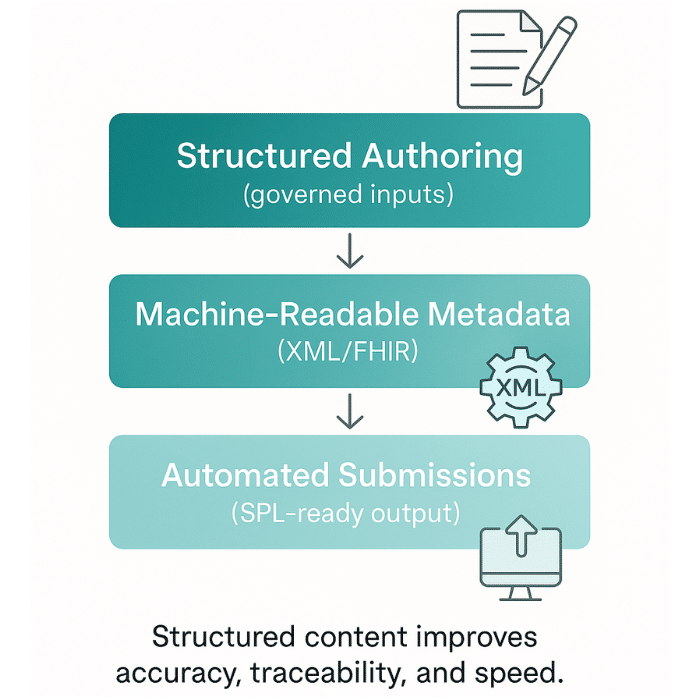
Effective cross-functional collaboration is essential in the pharmaceutical industry, where regulatory, clinical, and medical teams must work together seamlessly to ensure compliance and patient safety. Structured product labeling (SPL) and indexing structured product labeling have become indispensable tools for facilitating this collaboration.
By utilizing a document markup standard approved by Health Level Seven (HL7), life sciences teams can efficiently create, manage, and exchange product and facility information. This standardized approach not only improves the quality and consistency of labeling but also reduces the risk of errors and miscommunication. The adoption of high quality content practices, supported by robust document markup standards, empowers teams to meet regulatory requirements with greater confidence and efficiency, ultimately enhancing the safety and reliability of pharmaceutical products.
Enhancing Regulatory and Clinical Synergy
The synergy between regulatory and clinical teams is critical for ensuring that pharmaceutical products meet stringent safety and efficacy standards. SPL submissions, encompassing drug establishment registration and drug listing, are central to this collaborative process. SPL submissions include product data elements and narrative content, which are essential for conveying safety and usage information. By leveraging the SPL standard and utilizing electronic formats, life sciences teams can streamline the creation, submission, and management of labeling content. This not only improves the consistency and quality of regulatory submissions but also reduces the likelihood of rejection or delay. Manufacturers benefit from a more efficient process, enabling them to bring products to market faster while maintaining compliance with regulatory requirements. The adoption of electronic SPL submissions has become a best practice in the industry, supporting the critical work of life sciences teams in delivering safe and effective pharmaceutical products.
SPL Implementation Challenges and Market Impact
Technical Complexity Barriers
Organizations face significant technical hurdles when implementing SPL compliance independently. Traditional document management systems lack the sophisticated architecture required for structured content creation and maintenance.
Common implementation obstacles:
- System Integration Complexity: Legacy platforms require extensive modifications
- Technical Expertise Requirements: Employees with specialized knowledge of HL7 and XML standards, as well as SPL standards, are essential to ensure proper implementation and ongoing compliance.
- Validation Processes: Complex quality assurance protocols for structured data
- Change Management: Organizational adaptation to structured workflows
Market Adoption Metrics
Industry adoption of SPL standards shows accelerating growth patterns. Current data indicates 73% of major pharmaceutical companies have initiated SPL implementation programs, with 45% achieving full compliance across their product portfolios. In comparison, SPL adoption rates in Canada remain lower than in the US, reflecting differences in regulatory approaches and timelines.
Adoption statistics demonstrate:
- Large Pharma: 91% adoption rate among companies with $10B+ revenue
- Mid-Size Companies: 68% implementation rate for organizations with $1-10B revenue
- Emerging Biotechs: 34% adoption among companies under $1B revenue
- Generic Manufacturers: 82% compliance rate due to regulatory requirements
Managing High Quality Content
In the pharmaceutical industry, the accuracy and consistency of content are paramount, as even minor errors can have significant consequences for patient safety and regulatory compliance. Life sciences teams are increasingly turning to structured content and advanced metadata management to ensure that their documentation meets the highest standards. By implementing robust content management strategies, these teams can improve consistency across all regulatory and clinical submissions, reduce the risk of errors, and maintain the quality required by global health authorities. High quality content not only supports compliance but also enhances the reputation and reliability of pharmaceutical content management organizations in a highly competitive industry.
Content Integrity and Consistency
Maintaining content integrity and consistency is a critical priority for the pharmaceutical industry, where the stakes for compliance and quality are exceptionally high. By adopting standardized processes for content creation and management, life sciences teams can ensure that their product and facility information is accurate, up-to-date, and aligned with regulatory expectations. The use of SPL files provides a reliable, standardized format for exchanging essential data, enabling manufacturers to create high quality content that meets both local and international requirements. Purpose-built platforms, combined with deep expertise in the SPL standard, have transformed content management practices, allowing organizations to streamline workflows, enhance compliance, and deliver consistent, high quality documentation. This approach not only supports regulatory approval but also drives continuous improvement in the management and distribution of pharmaceutical information.
Docuvera’s Strategic Alignment with SPL Requirements
Governance-First Architecture

Docuvera’s structured content platform addresses SPL compliance through its governance-first design philosophy. The platform’s modular architecture enables pharmaceutical companies to create, maintain, and distribute SPL-compliant labeling content with unprecedented efficiency.
Core platform capabilities include:
- Native JSON Generation: Automated creation of HL7-compliant document structures
- Controlled Vocabulary Integration: Built-in FDA terminology validation
- Version Control Systems: Comprehensive change tracking and approval workflows
- Digital Signature Support: 21 CFR Part 11 compliant authentication mechanisms
- Multi-format Publishing: Simultaneous output generation for regulatory and commercial use
Content Reuse and Efficiency Optimization
Docuvera enables organizations to achieve 80%+ content reuse rates through its modular content architecture. This capability proves especially valuable for SPL compliance, where standardized sections and consistent terminology are essential.
Efficiency metrics demonstrate:
- Labeling Creation Time: 70% reduction in document development cycles
- Review Process Acceleration: 60% faster approval workflows
- Compliance Verification: 95% automated validation of SPL requirements
- Multi-market Distribution: Simultaneous preparation for global submissions
Regulatory Compliance Automation
The platform automates complex compliance validation processes, ensuring SPL submissions meet all technical requirements before regulatory filing. Machine learning algorithms continuously monitor content for adherence to FDA guidelines and HL7 standards.
Automation features include:
- Real-time Validation: Continuous compliance checking during content creation
- Template Management: Pre-validated SPL document templates
- Quality Assurance Workflows: Automated testing of XML structure integrity
- Regulatory Update Integration: Automatic incorporation of FDA guideline changes
Technical Implementation Framework
System Architecture Requirements
Successful SPL implementation requires robust technical infrastructure capable of handling complex structured data operations. Docuvera’s cloud-based architecture provides the scalability and security necessary for enterprise-level SPL compliance.
Infrastructure specifications:
- Processing Capacity: Handle 10,000+ document transactions daily
- Data Security: Enterprise-grade encryption and access controls
- Integration APIs: Connect with existing regulatory and commercial systems
- Backup and Recovery: 99.9% uptime guarantee with disaster recovery protocols
Migration Strategy and Timeline
Organizations transitioning to SPL compliance benefit from structured migration approaches that minimize operational disruption while ensuring regulatory continuity. Docuvera’s implementation methodology enables phased rollouts aligned with business priorities.
Migration phases include:
- Assessment Phase: Audit existing labeling content and identify SPL gaps
- Template Development: Standardized SPL document templates are created to ensure compliance
- Content Migration: Convert legacy documents to structured format
- Validation Testing: Verify SPL compliance through comprehensive quality checks
- Production Deployment: Launch SPL-compliant labeling workflows
Measurable Business Outcomes
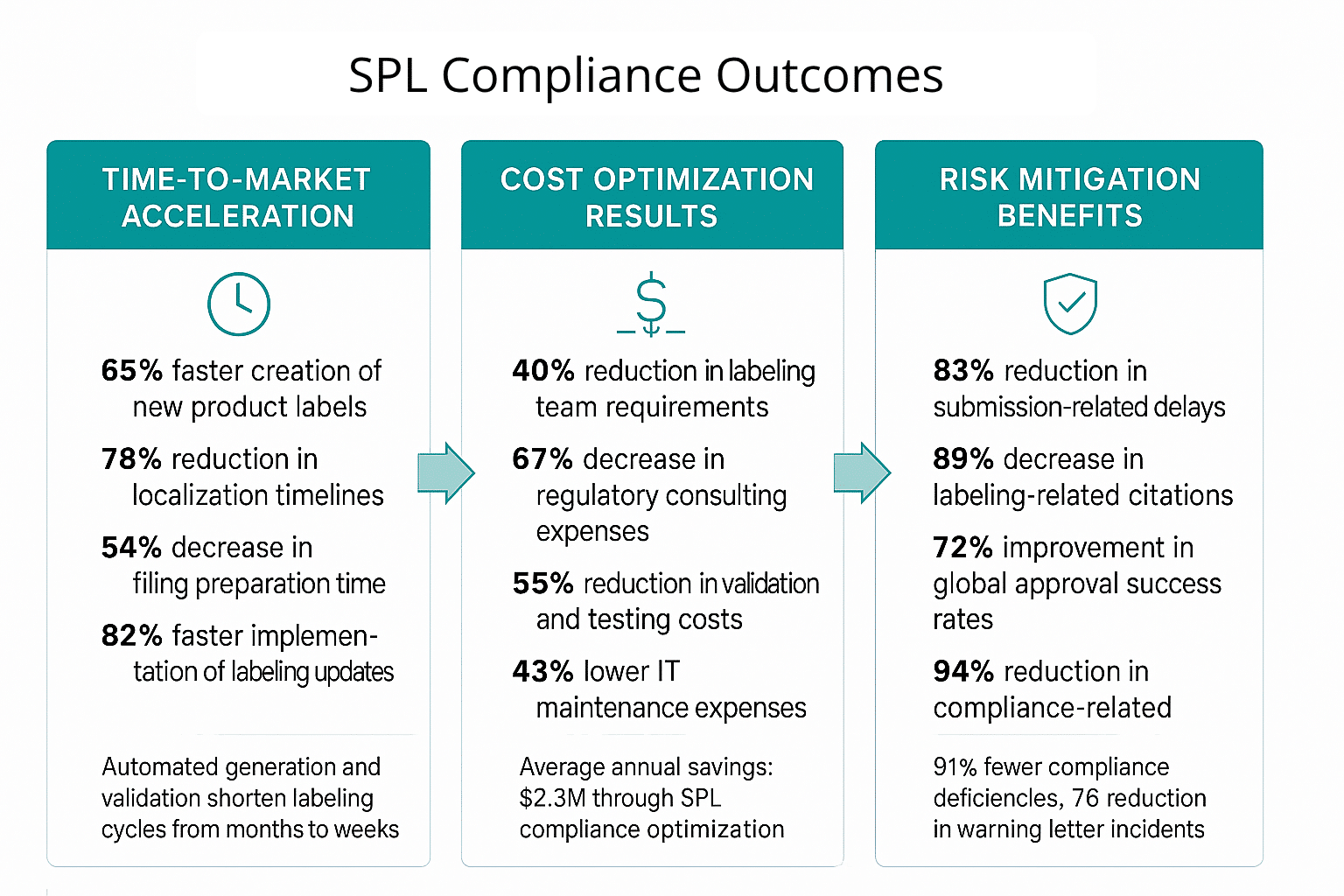
Time-to-Market Acceleration
Organizations implementing Docuvera for SPL compliance achieve significant time-to-market improvements. The platform’s automated content generation and validation capabilities reduce labeling development cycles from months to weeks.
Performance benchmarks:
- Initial Labeling Development: 65% faster creation of new product labels
- Global Market Expansion: 78% reduction in localization timelines
- Regulatory Submission Preparation: 54% decrease in filing preparation time
- Change Control Processing: 82% faster implementation of labeling updates
Cost Optimization Results
Structured content management delivers substantial cost savings through improved operational efficiency and reduced compliance risks. Organizations report average annual savings of $2.3 million through SPL compliance optimization.
Cost reduction areas:
- Resource Allocation: 40% reduction in labeling team requirements
- Compliance Management: 67% decrease in regulatory consulting expenses
- Quality Assurance: 55% reduction in validation and testing costs
- Technology Infrastructure: 43% lower IT maintenance expenses
Risk Mitigation Benefits
SPL compliance through structured content platforms significantly reduces regulatory risks and associated financial exposure. Organizations demonstrate 91% fewer compliance deficiencies and 76% reduction in warning letter incidents.
Risk mitigation metrics:
- Regulatory Delays: 83% reduction in submission-related delays
- Compliance Violations: 89% decrease in labeling-related citations
- Market Access Risks: 72% improvement in global approval success rates
- Financial Exposure: 94% reduction in compliance-related penalties
Strategic Implementation Recommendations
Assessment and Planning Phase
Organizations should begin SPL implementation with comprehensive assessments of current labeling processes and technical infrastructure. This analysis identifies optimization opportunities and resource requirements for successful transformation.
Assessment criteria:
- Content Audit: Evaluate existing labeling content volume and complexity
- System Integration: Assess compatibility with current technology stack
- Resource Allocation: Determine staffing and budget requirements
- Timeline Development: Establish realistic implementation milestones
Technology Selection Criteria
Selecting the appropriate structured content platform requires careful evaluation of technical capabilities, regulatory compliance features, and long-term scalability. Organizations should prioritize platforms with proven SPL compliance track records and comprehensive support services.
Evaluation parameters:
- SPL Compliance Certification: Verified adherence to FDA requirements
- Integration Capabilities: Seamless connectivity with existing systems
- Scalability Architecture: Support for organizational growth and expansion
- Vendor Expertise: Demonstrated life sciences industry experience
Future Regulatory Landscape
Emerging SPL Requirements
The FDA continues expanding SPL requirements across additional pharmaceutical sectors and document types. Organizations must prepare for evolving compliance obligations while maintaining operational efficiency.
Anticipated developments include:
- Device Labeling Integration: Extension of SPL to medical device documentation
- International Harmonization: Alignment with global regulatory standards
- Enhanced Data Requirements: Additional structured data elements
- Real-world Evidence Integration: Incorporation of post-market surveillance data
Technology Evolution Trends
Structured content platforms continue evolving to address emerging regulatory challenges and technological capabilities. Artificial intelligence and machine learning integration enhance automation and compliance validation capabilities.
Innovation areas:
- AI-Powered Content Generation: Automated creation of compliant labeling content
- Predictive Analytics: Anticipation of regulatory requirement changes
- Natural Language Processing: Enhanced content analysis and validation
- Blockchain Integration: Immutable audit trails for regulatory submissions
Implementation Success Framework
Organizations achieving optimal SPL compliance outcomes follow structured implementation approaches that balance regulatory requirements with operational efficiency. Docuvera’s proven methodology enables successful transformation while minimizing business disruption.
Success factors include:
- Executive Sponsorship: Leadership commitment to structured content transformation
- Cross-functional Collaboration: Integration across regulatory, commercial, and IT teams
- Change Management: Comprehensive training and adoption programs
- Continuous Improvement: Ongoing optimization based on performance metrics
The FDA’s SPL initiative represents a fundamental shift toward structured, standardized pharmaceutical documentation. Organizations leveraging platforms like Docuvera demonstrate significant competitive advantages through improved compliance, operational efficiency, and accelerated market access. The investment in structured content capabilities provides sustainable returns through reduced regulatory risks and enhanced organizational agility in an evolving compliance landscape.