For Chemistry, Manufacturing, and Controls (CMC) and regulatory leaders, confidence in regulatory outcomes depends less on the quality of any single submission and more on the organization’s ability to maintain leadership control over manufacturing knowledge over time. While the science may be rigorous, data complete, and processes validated, regulators increasingly evaluate how CMC knowledge is governed, reused, and defended across the product lifecycle and leadership is held accountable when that control breaks down. This is the control gap platforms like Docuvera are designed to address.
In this context, Module 3 is not just a content requirement. It represents a primary risk surface where gaps in governance surface during inspections, variations, and post-approval change discussions. Understanding its implications is therefore a leadership concern, not just a regulatory one.
CMC risk rarely originates in formulation design or process development alone. Instead, it accumulates downstream—when content leaves the hands of its original authors and begins to move across submissions, regions, and years.
What was once tacitly controlled by expert teams becomes distributed, reused, amended, and reinterpreted. Without structural governance, that risk compounds quietly, often unnoticed until regulatory scrutiny escalates and organizations are asked to explain—not just assert—control.
This shift reframes Module 3 as a determinant of regulatory control, not merely a container for CMC information.
Module 3 Evolution: From Submission Artifact to Lifecycle Asset
Module 3 of the Common Technical Document (CTD) was originally designed as a submission artifact—a structured snapshot of drug substance and drug product knowledge at a specific point in time. Its purpose was clarity and completeness for initial regulatory review. Over the past two decades, however, its role has expanded significantly.
CMC content is now evaluated twice: first at initial approval, and again each time a site, scale, specification, method, or control strategy changes post-approval. Initial approval establishes that commitments are acceptable as submitted. Lifecycle review determines whether those commitments remain coherent under change within the approved framework .
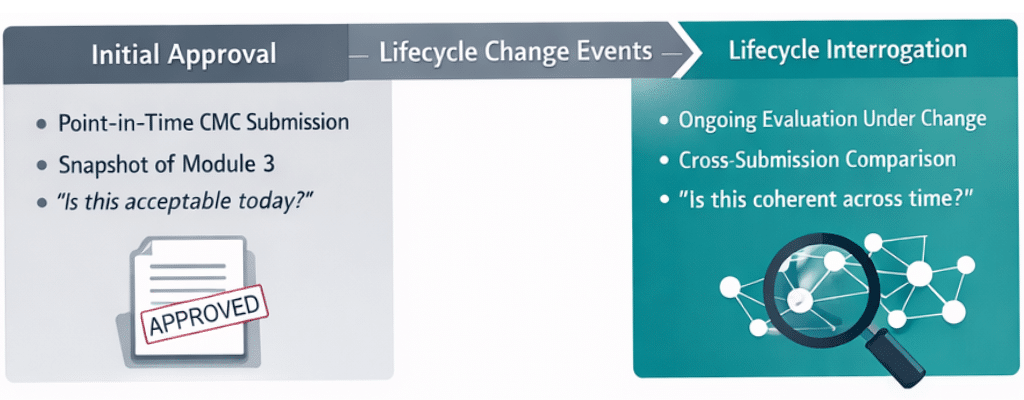
Today, Module 3 content functions as a long-lived operational asset. It underpins post-approval changes, global variations, site transfers, inspections, lifecycle management under ICH Q12, and emerging digital initiatives such as PQ-CMC. Regulators now expect consistency, traceability, and explainability of CMC knowledge across time and across markets—not just within a single dossier.
Regulatory direction, including FDA’s PQ-CMC and Knowledge-Aided Assessment and Structured Application (KASA), signals a shift from reading Module 3 as narrative to interrogating CMC commitments as lifecycle knowledge .
Yet this escalation in regulatory expectation has not been matched by a comparable shift in how most organizations govern CMC content. In many companies, Module 3 is still managed primarily as a collection of documents—updated, copied, and reassembled as needed. Control is applied at the document level, while the architecture of the content itself remains implicit, limiting the organization’s ability to demonstrate control during inspections.
This is where governed, component-based approaches—such as those supported by Docuvera—begin to matter.
The Leadership Problem Hiding in Plain Sight
As portfolios grow and globalize, reuse of CMC content becomes unavoidable. Drug substance descriptions are reused across strengths and dosage forms. Control strategies persist across sites and years. Manufacturing narratives carry forward through repeated regulatory interactions.
Reuse is not the problem. Unverifiable reuse is.
In practice, reuse often exists as copied paragraphs, inherited documents, and institutional memory. Teams may believe content is consistent because it “comes from the same source,” yet they often cannot demonstrate that consistency under inspection-level questioning—particularly when reuse spans products, markets, or time.
Variation work is where this fragility becomes visible first. Site additions, scale changes, analytical method updates, specification adjustments, and control strategy refinements force organizations to prove that existing commitments remain valid, bounded, and internally consistent under change . It is during these lifecycle events—not initial submission—that authority is tested.
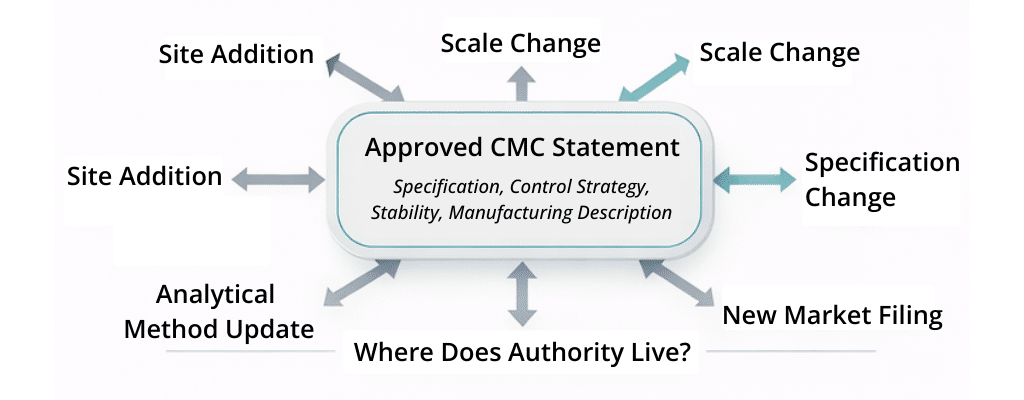
For leadership, this creates a compounding exposure. When inspectors probe lineage, consistency, or change rationale, organizations frequently rely on expert reassurance rather than system-level evidence. As inspection depth increases, the absence of governed reuse becomes an inspection liability—one that platforms focused on CMC content governance, such as Docuvera, are designed to reduce.
Why Document-Centric CMC Content Creates Regulatory Risk
At scale, managing CMC content primarily as documents introduces structural weaknesses that directly affect regulatory outcomes. Documents control files—versioning, approvals, and storage—but they do not control the knowledge architecture embedded within them.
From a leadership perspective, this leads to predictable consequences:
- Longer and more intensive inspections: Regulators probe deeper when reuse and consistency cannot be demonstrated clearly, extending inspection cycles and increasing scrutiny.
- Heavier change-management burden: Impact assessments rely on manual searches and cross-functional coordination, increasing cycle times and the likelihood of missed dependencies.
- Escalating internal review effort: Entire documents are repeatedly re-reviewed to compensate for uncertainty, increasing effort without proportionally reducing risk.
These outcomes are not theoretical. They are the operational signals that indicate CMC control still resides with people rather than systems—an imbalance that governed content platforms aim to correct.
At the root of this imbalance is where authority lives. In document-centric environments, authority remains attached to assembled artifacts—the last approved Module 3 section—rather than to the reusable CMC statement itself . When authority is document-bound, reuse must be re-proven repeatedly.
Regulatory Signals: Lifecycle Control Over Static Compliance
Recent regulatory guidance reinforces this direction. ICH Q12 explicitly frames CMC information as part of a managed lifecycle, emphasizing clarity around what is established knowledge, how changes are assessed, and how modifications are controlled post-approval.Similarly, initiatives such as FDA’s Pharmaceutical Quality–Chemistry, Manufacturing, and Controls (PQ-CMC) and the EMA’s digital transformation agenda signal a move away from narrative-only submissions toward structured, explainable CMC content.
These initiatives implicitly reward organizations that can demonstrate content lineage, reuse, and change history—capabilities that cannot be reliably achieved through document-centric approaches alone, but are core to platforms like Docuvera.
Why This Matters Now
Regulatory expectations are escalating faster than most CMC content operating models. As inspections become more data-driven and longitudinal, gaps in content governance translate into deeper questioning, extended inspection timelines, and higher remediation costs.
For CMC and regulatory leaders, the issue is no longer whether content is accurate. Accuracy is table stakes. The differentiator is whether content control is systemic or person-dependent.
Organizations that embed governance directly into CMC content—rather than layering process on top of documents—are better positioned to reduce inspection risk and stabilize regulatory outcomes. This is the shift Docuvera enables.
What Changes When CMC Content Is Governed at the Source
When CMC content is authored and managed as governed components, leadership outcomes shift.
Reuse becomes explicit rather than assumed. Change impact assessment becomes evidence-based rather than investigative. Review effort becomes proportional rather than defensive.
Authority relocates from the assembled document to the governed statement itself—with explicit scope, applicability, and lifecycle conditions . Once validity constraints (site, scale, lifecycle stage, stability dataset) are encoded structurally, impact analysis shifts from manual reconstruction to system behavior.
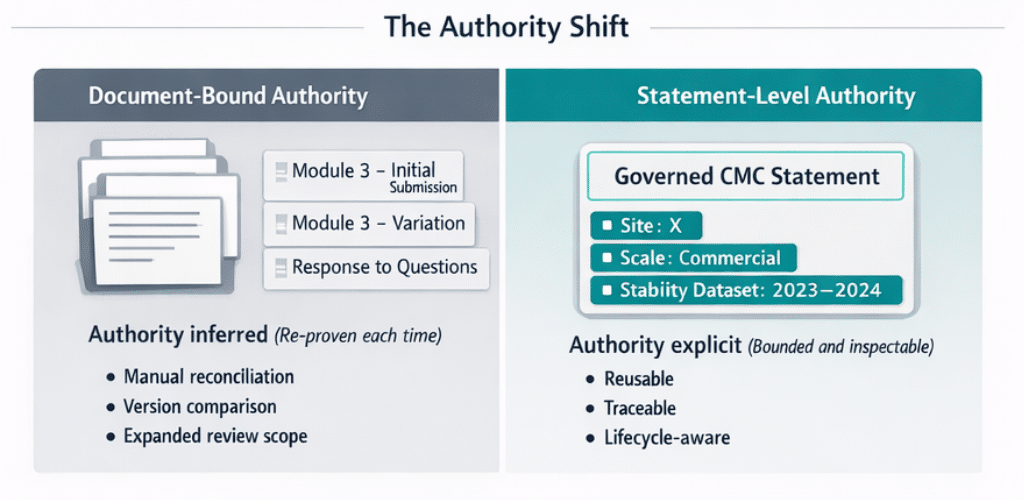
Most importantly, leadership can demonstrate control—showing inspectors where content is used, how it has changed, and how consistency is enforced—rather than relying on reassurance. This moves organizations from reactive inspection posture to confident regulatory engagement.
How Docuvera Supports Control
Docuvera provides a governance layer for CMC narrative content, treating it as a controlled enterprise asset rather than static text. By enabling component-level authoring, metadata, and traceability, Docuvera helps organizations shift control from people to systems.
This capability supports leadership objectives earlier in the regulatory lifecycle—before inspections, before variations, and before change-management complexity escalates—while remaining aligned with how CMC teams work today.
Next Steps
Assess where your current CMC control depends on people rather than systems—and where reuse cannot be demonstrated today. Identifying those gaps early is a leadership action, not a technology exercise.
Learn how Docuvera helps organizations strengthen CMC governance, reduce inspection risk, and improve regulatory confidence.