Pharmaceutical Quality–Chemistry, Manufacturing, and Controls (PQ-CMC) is often framed as a technical modernization effort—a move toward structured data, improved submission efficiency, and more digital interaction with regulators. That framing understates why leaders should care. PQ-CMC is best understood as a regulatory signal about control: how health authorities expect organizations to manage, explain, and defend manufacturing knowledge across the full product lifecycle.
This is not about whether information can be delivered in a new format. It is about whether an organization can stand behind the correctness, consistency, and governance of its CMC content—particularly narrative content—under scrutiny. As a result, PQ-CMC elevates CMC content governance from an operational concern to a leadership—and increasingly board-level—risk decision.
A Shift in How Regulators Assess Control
Historically, regulators evaluated CMC submissions largely as static artifacts. A Module 3 submission represented a snapshot of manufacturing knowledge at a specific point in time. While post-approval changes were expected and regulated, assessments focused on whether each submission was acceptable in isolation.
CMC is now evaluated twice: first at initial approval, and again each time the product, process, site, specification, or control strategy evolves post-approval. Initial approval establishes that commitments are acceptable as submitted. Lifecycle review determines whether those commitments remain coherent under change within the approved framework .
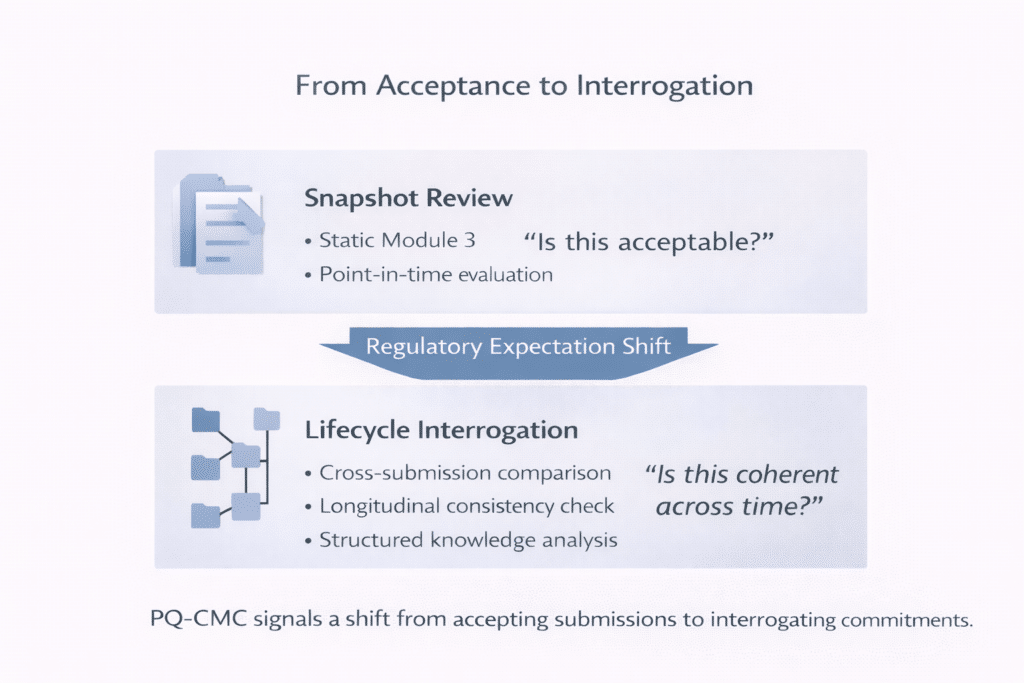
PQ-CMC reflects a clear shift away from this snapshot view. It aligns with a broader regulatory movement toward lifecycle management, predictability, and transparency—also visible in ICH Q12 and related initiatives. Regulators are no longer asking only what was submitted. They are testing whether organizations understand how manufacturing knowledge evolves, how changes are assessed, and whether content remains correct and consistent across submissions, products, sites, and markets.
This shift is reinforced by initiatives such as FDA’s Knowledge-Aided Assessment and Structured Application (KASA), which move review from narrative reconciliation toward lifecycle interrogation of structured quality knowledge . The regulator’s mental model is changing—from reading documents to interrogating commitments across time.
For leaders, this materially changes regulatory exposure. The question is no longer “How do you know?” but “Is this correct—and can you prove it across time?”
PQ-CMC as Pressure, Not a Specification
PQ-CMC is sometimes interpreted as a future technical specification. In practice, it functions as pressure. It signals that regulators expect organizations to understand their CMC information as a connected system of knowledge, not as a collection of documents assembled repeatedly under deadline.
Signals matter because they surface findings before formal mandates exist. Organizations that wait for prescriptive requirements risk discovering structural gaps during inspections. Organizations that treat PQ-CMC as directional pressure can address governance weaknesses before they become regulatory issues.
From a leadership perspective, the implication is direct: Can you defend CMC content as a governed system, not just as a series of approved files?
What PQ-CMC Implicitly Assumes Organizations Can Do
Although PQ-CMC discussions often emphasize structured data and digital review, the underlying assumptions are broader. PQ-CMC assumes organizations can:
- Identify where narrative CMC statements are reused across submissions, regions, and products
- Distinguish intentional reuse from accidental similarity
- Explain how changes propagate across dependent filings
- Demonstrate correctness and consistency without relying on manual reconciliation or expert memory
In many organizations, these capabilities exist informally. They reside in experienced authors and reviewers who remember what was reused and where. Control exists—but it is person-dependent rather than system-dependent.
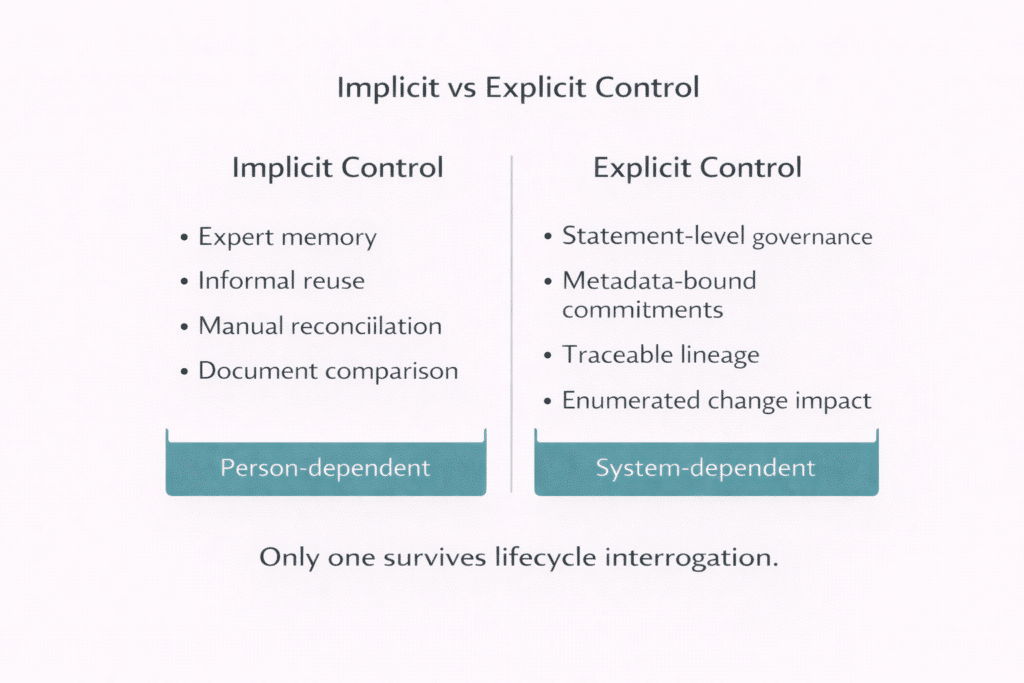
As portfolios expand and regulatory scrutiny deepens, this informality becomes fragile. When regulators probe across years of filings, answers must be reconstructed. That reconstruction introduces risk precisely when confidence and evidence matter most.
Variation work is where this fragility surfaces first. Site additions, scale changes, analytical method updates, specification adjustments, and control strategy refinements force organizations to prove that prior commitments remain valid, bounded, and internally consistent under change . It is during these lifecycle events—not during initial approval—that structural weaknesses in content governance become visible.
The System Gap Leaders Must Address
Most pharmaceutical organizations rely on a mix of RIM systems, document management platforms, and data repositories. Each plays a role, but none governs regulated narrative CMC content across its lifecycle.
Documents are approved, but relationships between content elements remain opaque. Reuse is inferred, not managed. Structured data may be tightly controlled, while narrative explanations—often the focus of regulatory questioning—remain embedded in static documents.
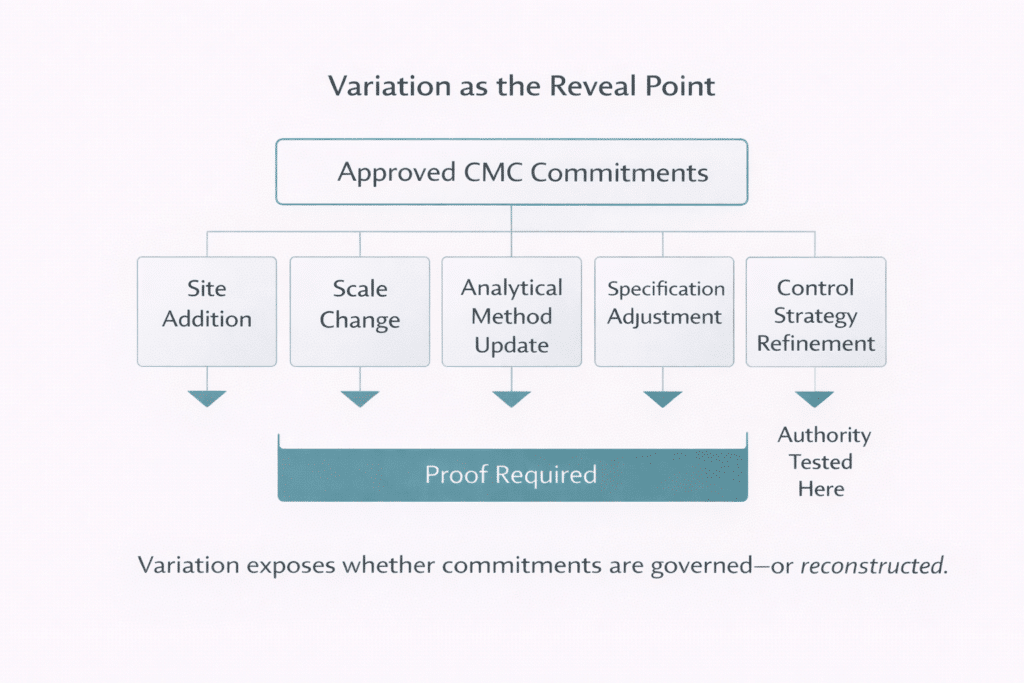
This creates a misleading sense of security. Submissions are filed. Inspections may pass. But when regulators ask whether a CMC statement is correct everywhere it appears, or how a change was assessed globally, leaders often fall back on manual searches and expert recollection.
Under PQ-CMC pressure, this gap becomes visible. Expectations shift from possession of information to demonstrable control over how that information behaves over time.
In practice, authority remains attached to assembled artifacts—the last approved Module 3 section or locally approved dossier—rather than to the reusable CMC statement itself . When authority is document-bound, reuse must be re-proven repeatedly. This is the structural mismatch PQ-CMC pressure exposes.
Why Retrofitting Structure Defers Risk
In response, some organizations focus on downstream conversion—extracting or structuring content after documents are written and approved. While this may satisfy short-term technical objectives, it separates governance from creation.
Narrative content is authored and reviewed in a document-centric context, then translated later. Inconsistencies, unintended reuse, and unclear change impact surface late, when timelines are compressed and options are limited.
From a leadership perspective, this does not reduce risk. It defers it. Assurance is rebuilt downstream instead of embedded upstream. As regulatory expectations intensify, deferred risk compounds across products, markets, and inspections.
Structure without relocated authority increases propagation without increasing control . Modularizing Module 3 narratives accelerates reuse, but if approval authority remains attached to assembled documents rather than governed statements, ambiguity scales with throughput.
Content Governance as Strategic Infrastructure
When CMC narrative content is governed at the source—as structured, metadata-rich components—control becomes durable rather than episodic.
Reuse is explicit. Relationships are visible. Change impact can be assessed before submissions are assembled. Organizations move from asserting consistency to demonstrating correctness with evidence.
Governance relocates authority from the assembled document to the reusable statement, with explicit scope, applicability, and lifecycle control . Once validity conditions—such as site, scale, lifecycle stage, or stability dataset—are encoded structurally, impact analysis shifts from manual reconstruction to system behavior.
For leaders, this shifts the control model. Predictability improves because content behaves in known ways across the lifecycle. Oversight replaces firefighting. This directly aligns with ICH Q12’s emphasis on predictability and effective lifecycle management. Without governed narrative content, those goals remain theoretical. With it, they become operational.
Inspection Dynamics and Explainability
PQ-CMC pressure will surface most clearly during inspections. Organizations that rely on manual explanation experience deeper questioning and extended follow-up cycles, as each answer triggers additional probes.
Organizations with governed CMC content respond differently. They can show where content is reused, how correctness is maintained, and how changes were assessed. Inspections shift from investigative to confirmatory.
For leadership, this difference is critical. Inspection outcomes increasingly affect regulatory trust, operational continuity, and corporate reputation. Explainability is no longer supportive—it is decisive.
How Docuvera Addresses the PQ-CMC Governance Gap
PQ-CMC does not mandate a specific technology. It exposes whether leadership claims about CMC control are defensible under scrutiny.
Docuvera was built to govern narrative CMC content as a lifecycle asset. Instead of treating content as static text embedded in documents, Docuvera manages it as structured, metadata-rich components with explicit reuse, lineage, and change awareness.
This enables what PQ-CMC implicitly demands:
- Explicit reuse rather than inferred similarity
- Systematic assessment of change impact before escalation
- Evidence-based explainability during inspections
- Lifecycle-level governance that persists beyond individual submissions
Docuvera complements RIM, EDMS, and data platforms by providing the missing governance layer for narrative CMC content—the layer PQ-CMC brings into focus. Content remains human-readable and workflow-aligned, while leadership gains durable, system-level control.
At a board level, this matters. PQ-CMC exposes whether CMC control depends on institutional memory or governed infrastructure. Docuvera converts PQ-CMC from uncertainty into predictability, reducing regulatory risk while strengthening inspection posture.
Future Readiness Is a Consequence of Governance
PQ-CMC is part of a broader trajectory toward automation, analytics, and responsible AI use in regulatory operations. All of these depend on the same foundation: governed content.
As articulated in lifecycle governance models, AI does not create control—it amplifies it . In document-centric environments, acceleration increases output without increasing provability. In governed environments, automation strengthens impact analysis, reuse discipline, and inspection readiness.
Without governance, automation amplifies inconsistency and AI magnifies uncertainty. With governance embedded upstream, innovation becomes safer and more predictable. Future readiness is not a separate initiative—it is a consequence of control.
Next Steps
Leaders should assess whether their CMC content architecture supports demonstrable correctness, explainability, and lifecycle control—or whether it relies on manual reconciliation and expert memory under pressure. Addressing this gap now determines whether PQ-CMC becomes a finding or a strategic advantage. Docuvera provides a governance-first foundation for meeting the standard regulators are now testing.
Get in touch with our team to learn more.
Endnotes
- U.S. Food and Drug Administration. Pharmaceutical Quality–Chemistry, Manufacturing, and Controls (PQ-CMC) Initiative. https://www.fda.gov/drugs/pharmaceutical-quality-resources
- International Council for Harmonisation (ICH). ICH Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management. https://www.ich.org/page/quality-guidelines
- European Medicines Agency. Regulatory science to 2025: strategic reflection. https://www.ema.europa.eu
- McKinsey & Company. Future RegTech and the potential of generative AI in regulatory submissions (Jan 2024). https://www.mckinsey.com
- Gartner. Structured Content Authoring as a Foundation for Regulatory Transformation in Life Sciences. https://www.gartner.com