Global labeling in the pharmaceutical space is one of the most regulated and resource-intensive areas in life sciences. Every label—whether for a small molecule drug or a biologic—communicates critical information about safety, efficacy, and proper use.
But here’s the reality: labels are never static. They evolve constantly as new clinical data emerges, regulatory requirements change, and market-specific adjustments come into play. Each of these updates has to be managed across multiple markets, in multiple languages, under strict compliance standards.
In this environment, how you create, manage, and distribute content directly influences compliance, operational efficiency, and ultimately, patient safety.
Structured content offers a better way forward.
The Traditional Labeling Dilemma
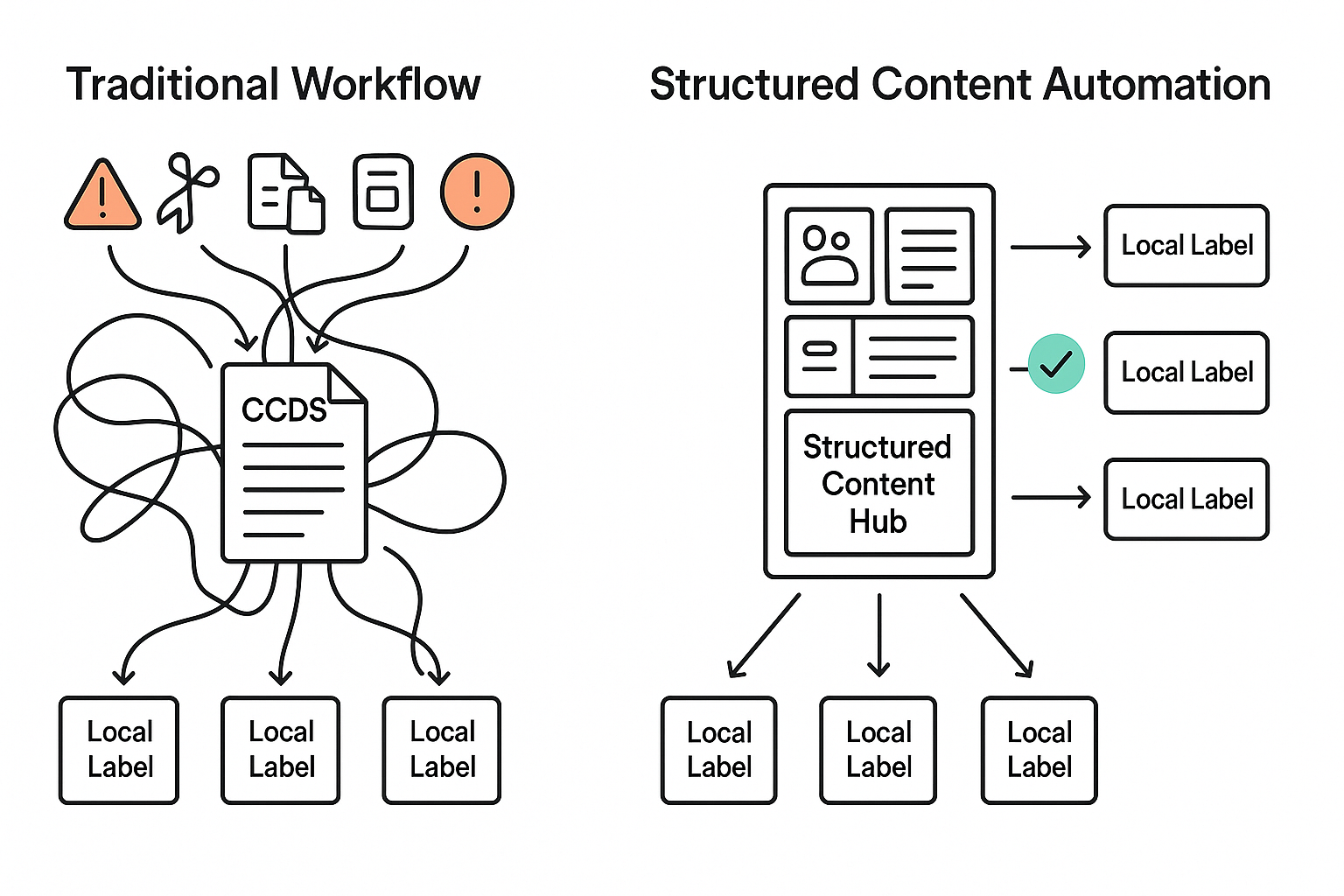
Most global labeling processes still revolve around documents. The Company Core Data Sheet (CCDS) serves as the foundation for regional product information (PI) and patient leaflets. From there, changes are distributed manually to dozens—or even hundreds—of local markets.
Each affiliate adapts the core text to meet local regulatory requirements, often through copy-paste workflows, tracked changes in Word, and a long trail of email approvals. While these methods have been the norm for decades, they introduce systemic risks:
- Fragmentation: Core updates don’t cascade consistently across all local labels.
- Version chaos: Tracking changes in spreadsheets and inboxes increases the likelihood of discrepancies.
- Compliance exposure: A missed or inconsistent update in one region can lead to regulatory findings or, worse, safety issues.
For companies managing large product portfolios across multiple regions, these inefficiencies scale quickly. What seems like “business as usual” becomes a real barrier to speed and compliance.
Why Inefficiency Matters to Pharma Leaders
Labeling inefficiencies are more than operational friction—they are strategic risks. Every delay in implementing a safety change extends the time before updated information reaches healthcare providers and patients. That delay can create compliance gaps, increase the chance of a regulatory citation, or trigger costly recalls.
There’s also a significant financial impact. Highly skilled teams spend hours performing manual, repetitive tasks: reformatting content, checking for inconsistencies, reconciling updates across documents. This drains resources that could otherwise be focused on strategic activities like accelerating submissions, improving patient access, and strengthening compliance frameworks.
In a global environment where speed, accuracy, and audit readiness are expected, clinging to document-based processes isn’t just inefficient—it’s unsustainable.
Structured Content: From Documents to Data
SCA replaces the traditional document approach with a modular one. Instead of managing entire documents, content is broken into components—discrete, reusable sections such as indications, contraindications, dosage instructions, and warnings.
Each component is tagged with metadata (e.g., region, language, regulatory status), making it possible to reuse and update content dynamically across multiple documents.
Why does this matter for labeling? Because much of labeling content is inherently repetitive and shared across markets. With structured content, an update to one component—such as a contraindication—can propagate to every relevant document and market, automatically flagging where local input is required.
It’s not just about efficiency. This approach improves accuracy and creates a controlled framework where global consistency and local variation can coexist.
The Business Case for Change
Structured content delivers measurable benefits that resonate at every level of the organization:
- Faster cycle times: Some companies report reducing labeling update timelines by 30–50%.
- Lower operational costs: Eliminating duplicate work frees resources for higher-value activities.
- Better audit readiness: A full version history at the component level simplifies regulatory inspections.
For organizations managing large portfolios across multiple markets, these efficiencies scale dramatically, translating into millions in savings and greater speed to market.
Global Alignment, Local Flexibility
One of the biggest challenges in global labeling is balancing standardization with local requirements. Structured content makes this possible.
Global teams can define canonical text for core safety statements, while affiliates can adapt that content for market-specific regulations within a governed process. All changes are tracked at the component level, creating transparency and reducing duplication of effort.
Other benefits include:
- Complete traceability of every change.
- Automation of consistency checks and translation workflows.
- Simultaneous multi-format publishing—from regional product information to electronic labeling—without manual reformatting.
The result? Fewer handoffs, fewer errors, and faster turnaround times across the entire labeling ecosystem.
The Regulatory Context: Why Now?

This shift isn’t happening in isolation—regulatory expectations are driving it. Global health authorities are moving toward greater transparency and structured, machine-readable data.
Initiatives such as IDMP (Identification of Medicinal Products), structured labeling submissions, and electronic product information (ePI) all signal that traditional document-centric processes are no longer future-proof. Regulators expect traceable, structured content that supports interoperability and data reuse.
Companies that make this shift now will not only reduce compliance risk but also avoid costly, last-minute overhauls as requirements evolve.
A Mindset Shift, Not Just a Technology Play
It’s tempting to view structured content as a tech implementation—but it’s more than that. It’s a cultural change, a departure from decades of document thinking toward a model where content is treated as data. Successful adoption requires buy-in across regulatory, labeling, medical writing, and IT teams. It demands governance, taxonomy, and new workflows.
- Content governance: Establishing taxonomies, metadata standards, and clear rules for content ownership.
- Technology enablement: Deploying a structured content platform that integrates with regulatory and labeling systems.
- Cross-functional collaboration: Engaging regulatory, labeling, affiliates, and IT teams early to align on workflows.
The payoff? A labeling process that’s not just compliant, but connected, efficient, and future-ready. Structured content and global labeling aren’t just complementary—they’re inseparable in the future of regulatory operations. The question isn’t whether pharma will make this shift. It’s how quickly.
How Docuvera Helps
Docuvera was built with the specific challenges of life sciences labeling in mind. Our structured content platform enables organizations to:
- Break down documents into reusable components with built-in metadata for easier global management.
- Automate content reuse and publishing across multiple formats and markets without manual rework.
- Ensure traceability and compliance with component-level version control and full audit history.
- Support regulatory readiness with capabilities designed for structured submission requirements.
By combining structured content technology with an intuitive user experience, Docuvera helps labeling and regulatory teams work faster, reduce risk, and maintain global consistency without sacrificing local flexibility.
Connect today to learn more about how our modern SCA solution can optimize your labeling process.