Why “structured” is the doorway—not the destination—for lifecycle-ready pharma content.
Pharma and biotech teams have spent years modernizing how they create documents. Templates are common. Structured documents are spreading. Some teams have even taken first steps into component-based authoring. Yet many organizations still struggle to make routine changes quickly, keep reused content consistent, and prove traceability across products and markets. The reason is simple: most transformations stop too early. “Structured” is a milestone—not the finish line. The SCA Maturity Continuum helps you see what comes next and how to get there.
Why this matters now
Regulators and the market are shifting from static, one-off submissions to continuous lifecycle management. ICH Q12 explicitly frames a globally harmonized approach for managing post-approval CMC changes predictably and efficiently throughout the product lifecycle—raising the bar on governance, data consistency, and change control.
In the EU, electronic Product Information (ePI) workstream activity and pilots aim to make authorized product information searchable, up-to-date, and usable across channels—driving expectations for structured, re-usable, and traceable content. Likewise, the EMA’s DADI (Digital Application Dataset Integration) initiative is replacing static PDF forms with web-based, data-driven processes aligned to ISO IDMP and related standards—another nudge toward governed, interoperable content that travels across systems.
Put bluntly: document-first operating models cannot keep up with lifecycle and data expectations. That’s exactly what the SCA Maturity Continuum surfaces—and solves.
What is the SCA Maturity Continuum?
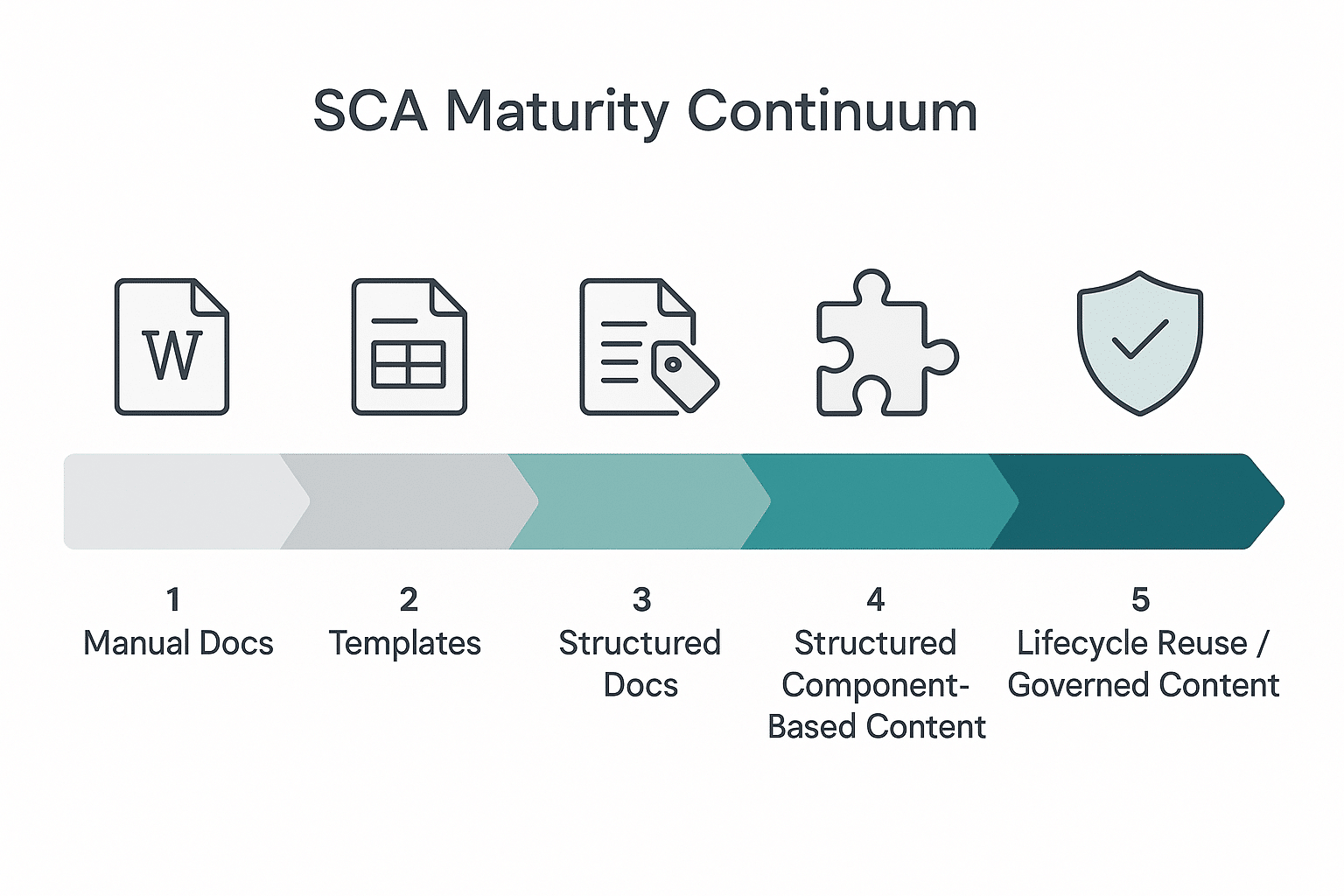
The continuum describes the evolution from manual, document-centric work to governed, lifecycle-ready content that can be safely reused across products, indications, regions, and submissions:
- Manual Docs | Teams draft in office tools, coordinate by email, and paste content from old files. Reuse is ad-hoc; traceability is fragile; cycle times are long.
- Templates | Branded templates standardize look and feel and improve consistency—but content remains copied, retyped, or “find-and-replace” updated. Processes still rely on people rather than systems.
- Structured Documents | Metadata appears, and some plugins or style guides help authors structure sections. But the unit of work is still the document, and re-review cycles persist because the same ideas are rewritten in many places.
- Structured (Component-Based) Content | Authors begin to assemble deliverables from reusable, approved components instead of starting from blank documents. This is a turning point—but without governance, component sprawl and uncontrolled reuse can still occur.
- Lifecycle Reuse (Governed Content) | Content becomes a governed, single source of truth with permissions, lineage, and audit trails. The same approved component flows safely into labels, CMC sections, clinical narratives, SOPs, and affiliate updates—without retyping. Changes propagate with visibility and control.
Most organizations we meet are clustered between stages 2–3, sometimes dabbling in stage 4. The business gains accelerate dramatically when you cross into stage 5, because governance transforms “structured” components into trusted components that can move through your ecosystem at speed.
How to place yourself on the continuum (quick self-assessment)

Ask your team these diagnostic questions and tally how many you can confidently answer “yes” to:
- Change propagation: When a core statement changes (e.g., a dosing instruction or CMC attribute), can you see every place it appears and update all instances without manual hunting? If not, you’re operating below stage 5.
- Ownership & permissions: Does each reusable component have clear ownership, approval status, and region/product applicability you can enforce at the system level—not just via SOPs? That’s governance (stage 5).
- Reuse at scale: Are authors guided by the system to reuse already-approved language instead of writing from scratch (with suggestions that respect product/market constraints)? That’s the jump from stage 3→4→5.
- Auditability: Can you trace who changed what, when, and why—across every deliverable fed by a component? That’s essential for lifecycle updates and aligns with ICH Q12’s predictability and change control expectations.
- Ecosystem fit: Can your content layer plug into RIM, QMS, EDMS, and affiliate systems so approved components flow through your stack rather than living in yet another silo? Mature stage-5 models complement—not replace—your ecosystem.
If you hesitated on more than two, you’re leaving time, quality, and compliance value on the table.
Crossing the chasm: from “structured” to governed content
The most common stall point sits between stages 3 and 4. Teams implement structured documents or even component-based authoring, but reuse still depends on people remembering where content lives, copying it correctly, and re-reviewing it repeatedly. To cross the chasm:
- Add a governance layer to define who can propose, approve, and update components by product, region, and team—with lineage and versioning by design.
- Embed an AI-powered reuse engine that suggests previously approved content components contextually—so authors spend more time deciding and less time hunting.
- Operate a single source of truth so the same governed component can be assembled into labels, 3.2.S/3.2.P sections, narratives, and SOPs—while staying synchronized.
This is not just a technology shift; it’s how you make “structured” operationally real in a lifecycle context—exactly where ICH Q12, ePI, and DADI are steering the industry.
What good looks like (and what slows you down)
Document-first friction points (stages 1–3):
- Core language gets recreated across teams and products.
- Change tracking depends on people, not systems.
- Author–review cycles add weeks, not hours.
Lifecycle-ready outcomes (stage 5):
- Dramatic reduction in re-review cycles because approved components are reused verbatim where appropriate.
- Fewer submission errors tied to reused content because lineage and applicability are visible.
- Faster affiliate updates with fewer escalations because the source component and its impact surface instantly.
These gains are consistent with external pressures: ePI requires up-to-date, accessible information across channels; DADI expects structured data alignment; and ICH Q12 calls for predictable post-approval change control. A governed component layer makes those outcomes repeatable, not heroic.
Your first moves: start small, scale fast
Organizations that accelerate through the continuum choose high-friction areas with obvious reuse, then expand. Common starting points include:
- Global Labeling: Assemble core statements once (safety, dosing, contraindications) and reuse with controlled variations across SmPC/USPI and local labels. ePI momentum makes structure and traceability especially valuable here.
- CMC Modules (3.2.S, 3.2.P): Stabilize master data and narrative alignment across changes; tie components to product and site applicability to align with lifecycle management expectations in Q12.
- Clinical Narratives: Reuse standard background and methodology language; protect variant sections with governance.
- SOP Libraries / Medical Information: Control frequent updates and downstream reuse at scale.
This phased, pragmatic approach is how teams move from “pilot islands” to enterprise impact.
What to expect from a purpose-built SCA platform
A mature SCA platform is more than a document editor. It provides:
- Structured, component-based creation with version control so authors assemble deliverables from governed building blocks.
- AI-powered content reuse that auto-suggests previously approved components in context.
- Governance and permissions configurable by team, product, and region to enforce who can change what.
- A single source of truth that exposes lineage and impact across formats and systems—RIM, QMS, EDMS, and affiliate portals—instead of creating yet another silo.
These capabilities are designed to complement—not replace—your ecosystem, creating the governed content foundation beneath it.
How to advance one step on the continuum—starting this quarter
- Map your high-value components. Identify the top 50 statements reused across labels, CMC sections, and market variations. (If you can’t list them, that’s your first gap.)
- Assign ownership and applicability. For each component, record product, region, and status—with a single approval path.
- Instrument change impact. Stand up lineage so you can answer, “If we update this, where else is it used?” before anyone edits.
- Pilot governed assembly. Build two deliverables (e.g., a core label update and a 3.2.P update) from the same governed components to prove propagation.
- Integrate with one upstream and one downstream system. For example, source product/site data from master data services and push approved text to your EDMS or affiliate portal. (This aligns with IDMP/SPOR data principles and avoids copy-paste drift.)
Each step moves you measurably along the continuum—without boiling the ocean.
The bottom line
If your transformation stopped at templates or structured documents, you’ve modernized the shell while leaving the engine untouched. The SCA Maturity Continuum makes the path visible: go beyond “structured” to governed, lifecycle-ready content that your teams and systems can trust. That’s how you cut weeks of re-review, reduce errors from reused content, and make every update—big or small—faster across markets. It’s not about one submission. It’s about every update.