The Emerging Trusted Regulatory Space in Life Sciences: Why It Matters and Who’s Shaping It
Introduction: The Case for a Trusted Regulatory Space
Digital transformation in life sciences is accelerating—and with it, the complexity of keeping regulatory data trusted, connected, and interoperable within what is increasingly referred to as a Trusted Regulatory Space (TRS). Across global teams, modernization and automation are outpacing the governance, traceability, and the policy frameworks needed to sustain them. Despite new technology and standards, most regulatory information still moves as documents, not as data. The tools are ready. The operating model is not.
What Is a Trusted Regulatory Space?
A Trusted Regulatory Space (TRS) is a secure, governed digital environment that enables sponsors and regulators to exchange structured, validated regulatory data rather than static documents. It integrates collaboration layers, regulatory information management systems, structured content platforms, and validation frameworks to ensure interoperability, traceability, and regulatory-grade security.
This disconnect stems from history. Regulatory workflows were built around static files, not structured data. Ownership was assigned to documents, not to the information within them. As a result, downstream teams still translate, reconcile, and revalidate the same content across systems, submissions, and markets—creating friction, duplication, and compliance risk that slow global operations and complicate interoperability across frameworks such as eCTD 4.0, IDMP, FHIR, and PQ-CMC.
That is why the concept of a Trusted Regulatory Space (TRS) is gaining traction, and quickly. A TRS creates a secure, governed ecosystem where sponsors, regulators, and partners exchange structured, validated, and trusted data instead of disconnected documents. Over the next 18 to 24 months, this transformation will redefine how the industry collaborates—accelerating submissions, improving transparency, and establishing the foundation for global digital trust in regulatory exchange.
What’s Emerging and Why Now
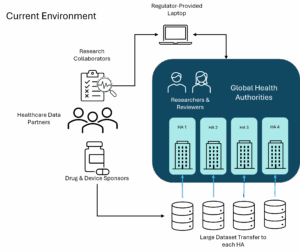 For decades, regulatory submission and review have followed a predictable sequence: sponsors compile, package, and send; health authorities receive, validate, and review. Even “electronic” submissions are essentially bundles of static files. To maintain integrity, this system depends on three core controls: a shared schema registry, explicit version alignment before exchange, and change-control procedures that allow targeted revalidation rather than rebuilding entire packages.
For decades, regulatory submission and review have followed a predictable sequence: sponsors compile, package, and send; health authorities receive, validate, and review. Even “electronic” submissions are essentially bundles of static files. To maintain integrity, this system depends on three core controls: a shared schema registry, explicit version alignment before exchange, and change-control procedures that allow targeted revalidation rather than rebuilding entire packages.
Digital transformation has modernized systems but not the structural bottleneck—the document-based nature of regulatory content. As global regulators shift toward structured, machine-readable, and interoperable data, the supporting infrastructure must evolve too.
Teams participating in pilot environments have learned that the human factor—training, coordination, and change tolerance—often determines success more than the tools themselves. Instead of sending documents back and forth, the TRS enables collaboration within secure shared environments, where regulators and sponsors can access, validate, and review information in real time. These interactions maintain compliance, traceability, and interoperability across frameworks such as eCTD 4.0, FHIR, IDMP, and PQ-CMC.
A useful example comes from oncology trials, where sponsor–regulator working groups use TRS principles to explore adaptive endpoints that balance speed with evidentiary rigor. Early pilots revealed challenges—schema version mismatches and metadata gaps—that highlight why TRS governance and regulatory data standards must evolve together.
Where Regulators Are Going
FDA (United States)
The FDA’s precisionFDA environment remains a secure, collaborative, cloud-based workspace demonstrating regulator–sponsor collaboration on structured data. Its workspace expansions have included live validation sessions showing how shared visibility accelerates issue resolution. The platform operates under the U.S. Government Cloud Security Program (FedRAMP), confirming its compliance and security posture, positioning precisionFDA as an operational model for TRS readiness.
Together, these initiatives illustrate a gradual but unmistakable convergence toward trusted digital exchange models, each moving at a different operational tempo but with shared intent.
EMA and the EU Network
The European Medicines Agency (EMA) is formalizing electronic Product Information (ePI) using FHIR-based structured data, aligning with evolving IDMP and SPOR data models to support pan-EU interoperability. This initiative represents Europe’s first major TRS-equivalent environment, promoting modular submissions, interoperability, and traceability between product master data, labeling, and regulatory planning. For sponsors, this convergence means fewer last-minute content discrepancies and smoother alignment of terminology across systems.
Global Collaboration Models
Collaborative frameworks such as the WHO Collaborative Registration Procedure (CRP) and CEPI’s multi-country review pilots illustrate how TRS-style models enable shared, interoperable regulatory processes across jurisdictions. These global efforts demonstrate the practical viability of trusted, multi-regulator collaboration spaces and highlight emerging models for secure regulatory data exchange.
The Importance of Structured Content Authoring in a TRS
Leading SCA solutions such as Docuvera are built for data compliance from the starting architecture. Conversely, the downstream systems your content is moving through may not be. Utilizing a TRS will amend that and, to gather the full value of other organizations, that content must be machine readable. This is best done from the start with structured component authoring (SCA), ensuring each modular component is metadata-rich, versioned, and interoperable across RIM and submission systems.
Signals and Near-Term Trajectory (12–24 Months)
The transition to TRS architectures is now visible across several fronts:
- From File Push to Shared Spaces: Regulatory workflows are shifting from one-way file submissions to dynamic collaboration environments featuring shared workrooms, embedded validators, and automated lineage tracking. Integration initiatives and expansions like precisionFDA point to this operational future.
- Regulator Onboarding as the Accelerator: Platform scalability depends on regulator adoption. The FDA is actively using TRS environments, while organizations are building multi-authority workspaces that connect sponsors and regulators across jurisdictions. Selective onboarding will create the first network effects.
- RIM as Orchestration Layer: Regulatory Information Management (RIM) systems are evolving from systems of record to systems of orchestration, reflecting broader RIM modernization efforts across the industry. They increasingly connect structured-content and collaboration platforms, ensuring that validated, traceable data flows seamlessly across regulatory and compliance domains. In reality, many organizations are still learning how to reconcile governance ambition with day-to-day operational pressure—a balancing act that exposes both technical and cultural gaps.
- Standardization Accelerators: The maturation of eCTD 4.0 and FHIR-based ePI frameworks enables API-centric data exchange. Platforms capable of streaming structured content rather than static documents will have a competitive edge as interoperability becomes expected.
- Security and Sovereignty: ISO/IEC 27001:2022, Annex 11, and FedRAMP equivalence models are becoming prerequisites for participation in TRS environments. Where local requirements diverge, multi-jurisdictional TRS infrastructures employ policy abstraction—one core model with configurable national enforcement.
The TRS Ecosystem and Roles
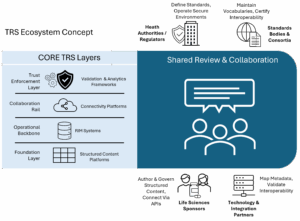 Key Layers of the Trusted Regulatory Space Architecture
Key Layers of the Trusted Regulatory Space Architecture
The TRS is not a single technology—it is an ecosystem composed of interoperable layers and participants aligned under shared governance.
Core Technology Layers
- Connectivity Platforms (The Collaboration Rail): These secure environments enable live interaction between regulators and sponsors, ensuring real-time validation, data segregation, and auditability under shared frameworks.
- Regulatory Information Management Systems (The Operational Backbone): RIM systems govern product master data, submission planning, and compliance tracking. They act as control centers that connect internal workflows to external TRS environments.
- Structured Content Platforms (The Foundation Layer): Structured authoring transforms traditional document creation into modular, machine-readable components. Each piece carries metadata and validation rules aligned with standards like eCTD, FHIR, and PQ-CMC, ensuring traceability and reuse across submissions.
- Validation and Analytics Frameworks (The Trust Enforcement Layer): These tools verify schema compliance, monitor interoperability, and provide AI-assisted quality control under auditable governance conditions.
Participant Roles
- Health Authorities and Regulators: Define structured standards, operate secure collaboration environments, and oversee multi-authority reliance pilots.
- Life Sciences Sponsors: Serve as data originators and custodians, implementing modular authoring, metadata governance, and secure API integration with regulatory partners.
- Technology and Integration Partners: Map metadata and data models across legacy systems, validate interoperability, ensure regulatory-grade compliance, and maintain data lineage across distributed systems..
- Standards Bodies and Consortia: Maintain shared vocabularies, technical specifications, and certification programs that anchor interoperability across global jurisdictions.
The interplay among these participants defines the real measure of TRS maturity—alignment is less about perfect standards and more about shared operational trust and cross-jurisdictional interoperability.
Why It Matters and What’s Next
From direct participation in structured-data pilot programs between 2023 and 2024, several teams observed that the operational burden shifts from publishing to governance. This experiential insight grounds the subsequent benefits outlined below.
It’s worth admitting that digital transformation, when driven by compliance deadlines rather than design intent, can stall genuine interoperability. We’ve witnessed that tension repeatedly in joint-review pilots.
- Digital Transformation with Impact: Instead of modernizing interfaces alone, TRS models address the core structural issue—unstructured, siloed data—by embedding governance, validation, and traceability from creation.
- True Sponsor–Regulator Collaboration: Shared access and real-time validation shorten review cycles, support submission automation, reduce redundant queries, and foster trust between stakeholders.
- Governance and Auditability by Design: The TRS embeds compliance and lifecycle tracking at the point of authoring, ensuring that every change and version remains auditable.
- AI and Advanced Analytics Readiness: AI can only operate safely on structured, validated data. TRS governance frameworks make AI use traceable and regulator-compliant.
- Global Consistency and Public Trust: With structured interoperability, a single update to labeling, safety, or clinical data can cascade across jurisdictions instantly, maintaining accuracy and protecting patients.
Next Developments include expanded regulator participation in structured data pilots, multi-authority review programs, harmonized APIs and metadata models, deeper system integration, federated security models (Regulator-grade certifications (FedRAMP, ISO 27001, Annex 11 equivalence)), and a transition from existing pilot programs to production-scale adoption.
How to Start: Practical Pathways to TRS Readiness
Steps organizations can take to prepare for regulatory interoperability.
Organizations can begin preparing for TRS participation by embedding governance, interoperability, and structure into daily operations.
- Structure the Source: Move away from document-first models. Establish a governed, modular content architecture where every component is metadata-rich, validated, and interoperable at creation. This structured foundation is the single most critical enabler of TRS participation.
- Strengthen Governance and Traceability: Implement governance frameworks that enforce version control, audit trails, validation checkpoints, and content lineage across systems. Ensure every data element, document, and exchange is fully traceable and interoperable.
- Integrate Strategically: Connect authoring, RIM, and submission systems through secure, interoperable APIs designed for regulatory cloud environments. Eliminate manual file transfers. Enable continuous, standards-based data exchange between internal and external environments.
- Engage in Collaborative Pilots: Participate in regulator or industry TRS initiatives to gain early experience, validate interoperability, and refine governance models before large-scale adoption.
- Align Cross-Functional Teams: Ensure that regulatory, IT, quality, and compliance functions operate under unified governance principles — emphasizing structure, data integrity, and interoperability from the outset.
Readiness Considerations
 Ask these questions to assess maturity:
Ask these questions to assess maturity:
- What is the maturity of your structured content and data governance framework today — and how quickly could it interoperate with external TRS environments?
- Are your regulatory, quality, and clinical teams aligned on metadata standards and content models, or are they still operating in silos?
- Do your compliance policies embed governance at content creation?
- How will you validate interoperability between internal systems and external TRS platforms?
- How will your existing RIM and submission systems connect to the external collaboration and validation layers that define the TRS?
- Do your compliance policies ensure that governance and structure begin at the point of authoring, not downstream during publishing?
- Are you designing your workflows to anticipate regulator access to shared environments — and ensuring security, segregation, and auditability accordingly?
- How will you validate interoperability between your internal systems and external TRS environments before regulators make it mandatory?
The Bottom Line
The TRS is not a product—it is a governance architecture that fuses structured content, interoperable systems, and regulator collaboration into one trusted digital ecosystem. When these elements converge, the benefits are tangible: faster submissions, transparent reviews, and higher confidence in outcomes. TRS represents the scaffolding of digital trust that underpins the future of regulatory innovation.
For practitioners shaping this evolution, the TRS is more than an infrastructure—it’s a test of collective readiness to embed trust into every layer of digital collaboration.
Top Trusted Regulatory Space (TRS) FAQs
Compliance with eCTD 4.0, FHIR-based ePI, SPL, and IDMP is essential. Sponsors must demonstrate modular, machine-readable content, validated lineage, and controlled vocabularies.
Validation relies on technical conformance guides, standardized API schemas, and shared validation engines supporting XML/JSON structured data—aligned with ICH eCTD v4.0 implementation specifications and HL7’s FHIR conformance resources.
Through immutable audit trails, cryptographic validation, synchronized version control, and role-based access matrices—as outlined in ISO/IEC 27001:2022 and Annex 11 requirements for computerized systems.
Continuous validation and lifecycle management ensure that every change remains auditable and propagated correctly across linked submissions. This approach mirrors the FDA precisionFDA collaborative model, which emphasizes real-time validation and traceability.
Regulatory and IT professionals will need skills in structured-content management, metadata governance, and interoperable system integration—especially as frameworks like FHIR and IDMP converge across jurisdictions.
It enhances transparency by providing real-time audit trails and provenance data accessible to regulators, consistent with the expectations outlined in EU GMP Annex 11 and ISO 13485 for quality management systems.
Ongoing metadata governance, harmonized taxonomies, validated reuse rules, and persistent encryption at rest and in transit—all aligned with ISO/IEC 27001, FedRAMP, and data-integrity guidance from regulators such as the EMA and FDA.