Introduction: Why a Trusted Regulatory Space Is Becoming Necessary
Digital transformation in life sciences is accelerating—but regulatory operations remain constrained by a legacy reality: most regulatory information still moves as documents, not as data. Even as organizations invest in new platforms, automation, and AI, the underlying operating model has not kept pace.
Regulatory workflows were historically designed around static files. Ownership was assigned to documents, not to the information within them. As a result, the same content is repeatedly translated, reconciled, and revalidated across systems, submissions, and markets. This creates friction, duplication, and compliance risk—precisely where regulators expect greater speed, transparency, and traceability.
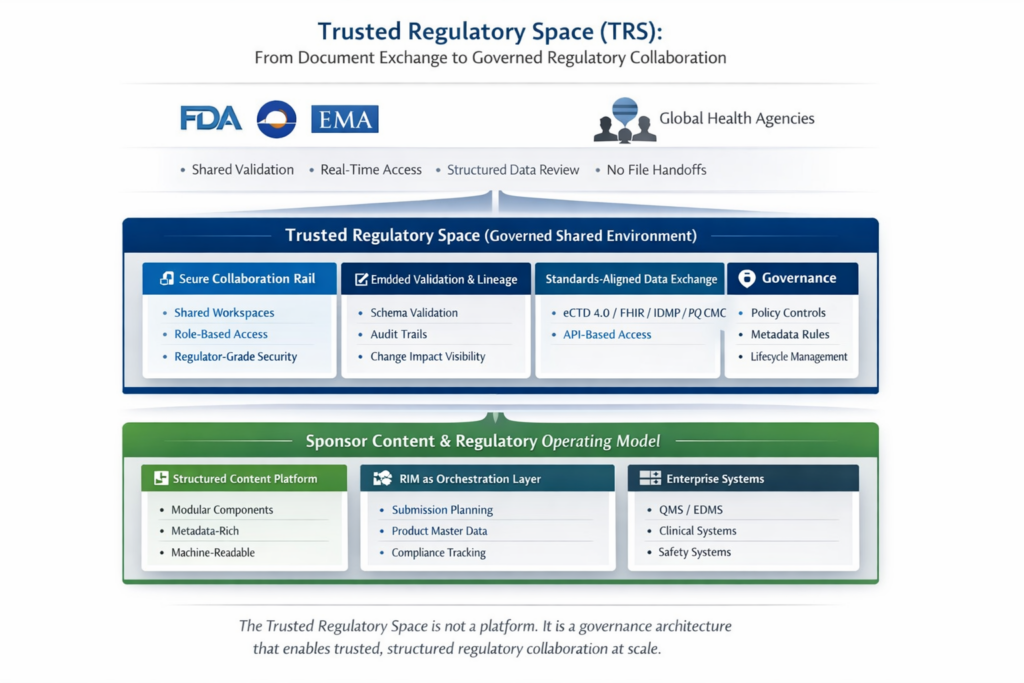
This disconnect is why the concept of a Trusted Regulatory Space (TRS) is gaining traction. A TRS is not a tool or a portal. It is a secure, governed ecosystem in which sponsors, regulators, and partners exchange structured, validated, and trusted data rather than disconnected document packages.
Over the next 18–24 months, this shift will begin to redefine regulatory collaboration—changing how submissions are reviewed, how updates propagate globally, and how trust is established in digital regulatory exchange.
Why Now: What’s Driving the Shift
For decades, regulatory exchange followed a predictable pattern: sponsors compiled submissions, packaged them, and sent them to authorities for review. Even “electronic” submissions were still collections of static files. Integrity depended on manual controls—version alignment, schema checks, and extensive downstream revalidation.
While systems have modernized, the structural bottleneck has remained. Regulatory content is still created and managed as documents, even as regulators move toward structured, machine-readable, and interoperable data.
Pilot programs have shown that the biggest challenge is not tooling—it is governance. Teams quickly discover that success depends on shared standards, version control, metadata discipline, and clear ownership of data elements. In this context, a Trusted Regulatory Space becomes the environment where governance is enforced operationally, not retroactively.
Instead of sending files back and forth, sponsors and regulators collaborate within secure shared spaces. Validation occurs in real time. Lineage is preserved. Changes are reviewed at the data level rather than by rebuilding entire submissions. These environments align with emerging frameworks such as eCTD 4.0, FHIR-based ePI, IDMP, PQ-CMC, and ICH M11, all of which assume structured content as a prerequisite.
Where Regulators Are Leading
United States: FDA
The FDA’s precisionFDA initiative provides a concrete signal of TRS direction. precisionFDA is a secure, cloud-based collaborative environment that enables regulators and sponsors to work directly with structured data in shared workspaces, accelerating issue resolution through live validation and shared visibility. Its operation under U.S. government cloud security programs reinforces that trust, security, and governance are foundational to digital regulatory collaboration.
https://www.fda.gov/about-fda/office-digital-transformation/precisionfda
In parallel, the FDA continues to advance structured submission standards, including eCTD 4.0, which supports message-based, data-driven regulatory exchange rather than static file delivery. https://www.fda.gov/media/179700/download
Europe: EMA and the EU Network
The European Medicines Agency is formalizing electronic Product Information (ePI) using FHIR-based structured data, marking one of the clearest TRS-equivalent implementations to date. ePI enables modular submissions and traceable alignment between product master data, labeling, and regulatory planning.
https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/product-information-requirements/electronic-product-information-epi
The associated FHIR implementation guidance further reinforces expectations for machine-readable, interoperable product information.
https://build.fhir.org/ig/HL7/emedicinal-product-info/
For sponsors, this convergence reduces last-minute discrepancies and improves consistency across markets. More importantly, it signals that regulators expect information to be query-able, traceable, and reusable, not just readable.
Global Collaboration Models
Initiatives such as the WHO Collaborative Registration Procedure and multi-country review pilots illustrate how TRS principles enable shared regulatory reliance across jurisdictions. These efforts demonstrate that trusted, multi-authority collaboration is operationally feasible when governance and structure are aligned.
What the Next 12–24 Months Will Bring
Several clear signals indicate where TRS architectures are heading:
From File Transfer to Shared Spaces
Regulatory workflows are shifting from one-way submissions to shared collaboration environments with embedded validators, auditability, and automated lineage tracking.
Regulator Onboarding as the Accelerator
The real inflection point will be regulator participation. As authorities onboard into shared environments, network effects emerge—making structured exchange the expected norm rather than a pilot exception.
RIM as an Orchestration Layer
Regulatory Information Management systems, such as EXTEDO, are evolving from systems of record into systems of orchestration—connecting structured authoring platforms, collaboration environments, and submission channels under a unified governance model.
Standards as Enablers, Not Endpoints
The maturation of eCTD 4.0, FHIR, and API-centric frameworks enables streaming of structured content rather than static document handoffs. Platforms that cannot support this shift will become bottlenecks.
Security and Sovereignty by Design
Participation in TRS environments increasingly presumes compliance with frameworks such as 21 CFR Part 11 and EU Annex 11, where audit trails, access control, and data integrity are mandatory rather than optional. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-11
The Trusted Regulatory Space Ecosystem
A TRS is not a single technology. It is an ecosystem of interoperable layers aligned under shared governance.
Connectivity and Collaboration Platforms
Secure environments where regulators and sponsors interact in real time, with segregation, validation, and auditability enforced by design.
Regulatory Information Management (RIM)
The operational backbone governing product data, submission planning, and compliance tracking—connecting internal workflows to external regulatory spaces.
Structured Content Platforms
The foundation layer where content is authored as modular, machine-readable components enriched with metadata and validation rules aligned to regulatory standards.
Validation and Analytics Frameworks
Trust enforcement layers that verify schema compliance, monitor interoperability, and support AI-assisted quality control within auditable boundaries.
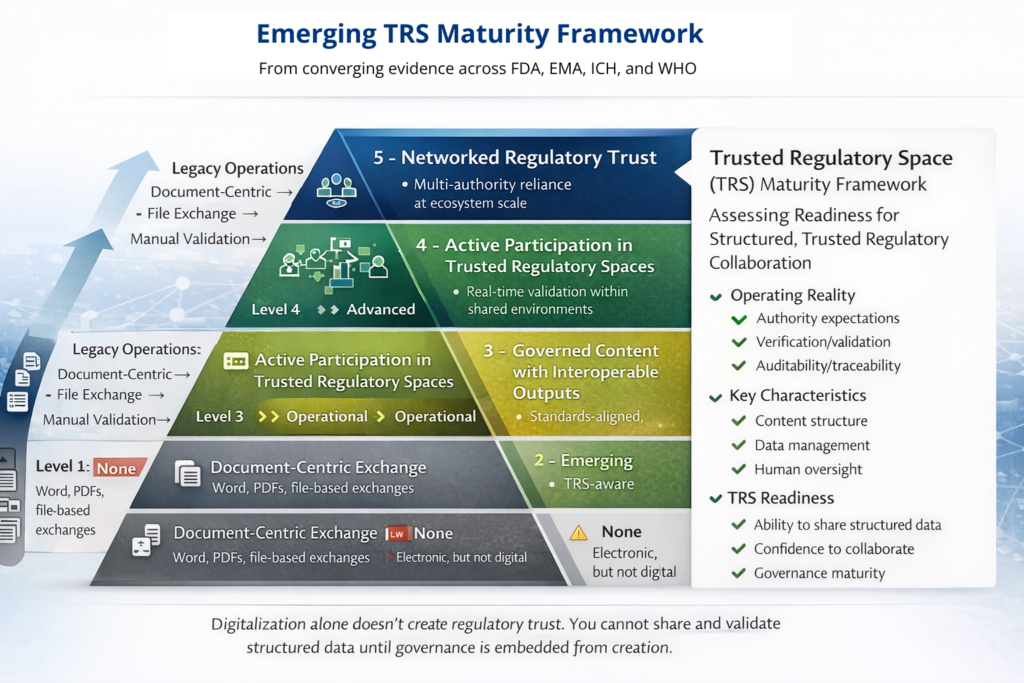
Across these layers, maturity is measured less by perfect standards and more by operational trust—the ability to share, validate, and reuse data confidently across jurisdictions.
Why It Matters
Experience from structured-data pilots shows a consistent pattern: as organizations move toward TRS participation, effort shifts from publishing to governance. This is not a drawback—it is the point.
Digital Transformation with Real Impact
TRS models address the root problem: unstructured, siloed content. Governance, validation, and traceability are embedded at creation, not imposed downstream.
True Sponsor–Regulator Collaboration
Shared access and real-time validation reduce redundant queries, shorten review cycles, and build confidence on both sides of the regulatory interface.
Auditability by Design
Every change, version, and dependency is tracked at the data level, supporting inspection readiness without manual reconstruction.
AI Readiness with Boundaries
AI can only be trusted when operating on structured, validated data. TRS governance frameworks make AI use traceable, reviewable, and regulator-appropriate.
Global Consistency and Public Trust
When updates cascade across markets from a single governed source, accuracy improves—and patient trust is protected.
How Organizations Can Prepare Now
TRS readiness does not begin with external pilots. It begins internally.
Structure the Source
Move away from document-first models. Establish a governed, modular content architecture where every component is validated and metadata-rich at creation.
Embed Governance Upstream
Ensure version control, audit trails, and lineage are enforced where content is authored—not after it is assembled.
Integrate, Don’t Transfer
Connect authoring, RIM, and submission systems via secure APIs. Eliminate manual file handoffs wherever possible.
Engage Early
Participate in regulator and industry pilots to test interoperability and governance models before mandates arrive.
Align Teams Around Data Stewardship
Regulatory, quality, IT, and compliance teams must operate under shared principles that prioritize structure, integrity, and interoperability.
The Bottom Line
The Trusted Regulatory Space is not a product. It is a governance architecture that brings together structured content, interoperable systems, and regulator collaboration into a single, trusted ecosystem.
As regulators move from documents to data, TRS models provide the scaffolding for digital trust—enabling faster submissions, clearer reviews, and higher confidence in outcomes.
For organizations shaping the future of regulatory operations, the question is no longer whether this shift will happen—but whether their content, governance, and operating models are ready to participate.