What Structured Content Really Looks Like in Pharma
Structured content authoring (SCA) is often described in terms of benefits—faster reviews, better reuse, multiformat publishing. But for many regulatory, medical, and labeling professionals, the concept remains abstract.
What does structured content actually look like in practice?
What defines a content component?
And how does this apply to the real-world documents your team manages every day?
It’s time to shift from document thinking to content strategy.
If your teams are still working with full-document files, it’s time to rethink what’s possible.
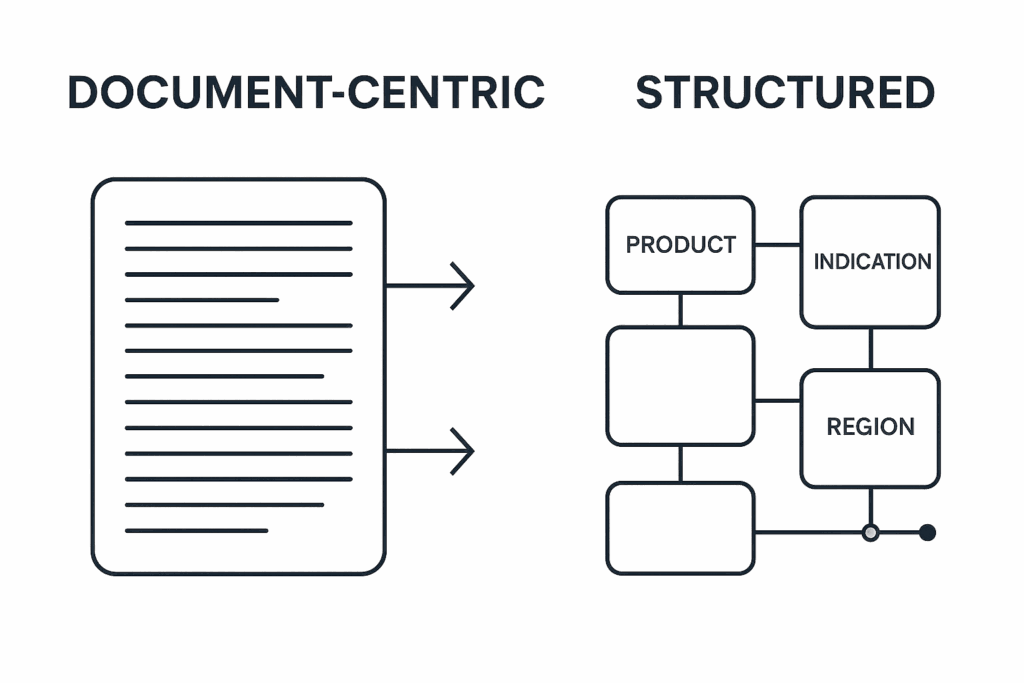
From Documents to Reusable Components: A Necessary Shift
Most regulatory and medical content is still managed in entire documents—Word files or PDFs organized by region, product, or submission. These documents often contain repeated language: product descriptions, dosage instructions, risk statements, or regulatory justifications.
Structured content authoring deconstructs these documents into their reusable parts—and manages each one as an independent, version-controlled unit. These governed units are referred to as content components or building blocks.
Instead of writing a submission from top to bottom, you assemble it from approved components.
What Defines a Content Component?
A content component is a discrete unit of content that meets the following criteria:
Purpose-driven (e.g., “Storage Conditions Statement”)
Reusable in multiple documents or formats
Metadata-tagged (e.g., product, market, indication, language)
Independently reviewed, versioned, and traceable
Centrally updated, with changes cascading automatically across uses
These components can take many forms:
A single paragraph
A structured table
A heading with nested bullet points
A localized variation of a global statement
The optimal size depends on how often it’s reused, how frequently it changes, and how traceable it needs to be.
Example: What a Structured Content Component Looks Like
Here’s a simplified example of how a single content component is structured and reused:
Component Name: Dosage Instruction – Pediatric
Content: “For children aged 6–12 years, administer 5 mL once daily.”
Metadata Tags: Product A, Europe, Labeling, SmPC, EN, Pediatric
Usage: Referenced in 7 SmPCs, 3 PILs, 2 ePI submissions
When this instruction changes, the update is automatically reflected across all dependent documents—ensuring speed, accuracy, and regulatory alignment.
Where Do Content Components Fit in the Workflow?
Structured content platforms like Docuvera allow regulatory and medical teams to:
Author new components using guided templates
Reuse approved components across multiple assets
View a full reuse map of each component
Track approval status and version history
Push updates that automatically reflect across all connected documents
In practice, a full SmPC or submission document may be constructed from 20–50 structured content components—some product-specific, others global standards reused across therapeutic areas.
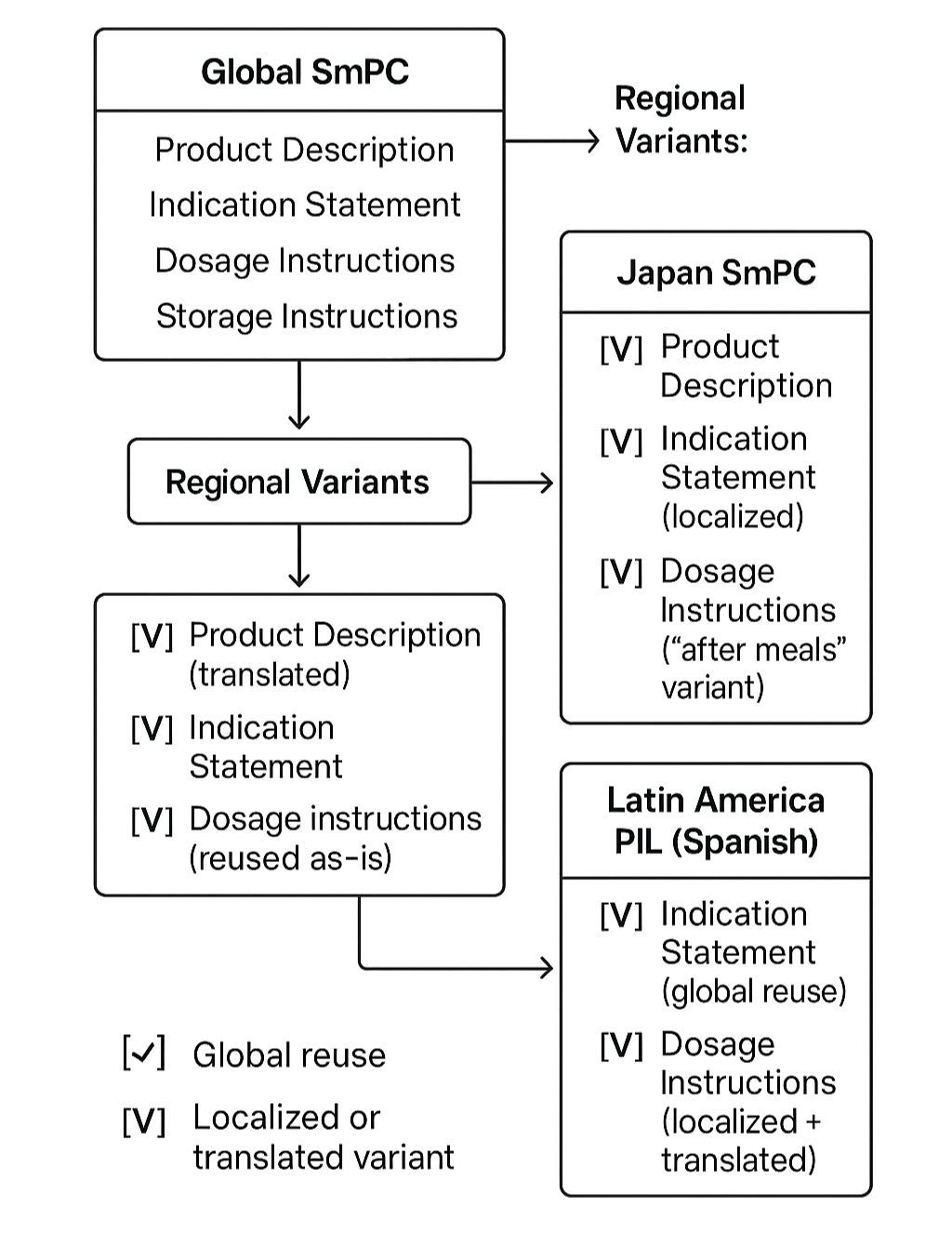
How Structured Components Support Reuse and Localization
Structured content components can be reused across documents and adapted through variants. Here\’s a simplified illustration of how one set of components supports multiple global and local outputs:
What Makes an Effective Content Component?
Not all content lends itself to structured reuse. Good components are:
Self-contained – They communicate a single purpose or message.
Context-aware – Metadata guides their appropriate use.
Reusable – They are relevant across multiple outputs or regions.
Traceable – They link directly to their data or regulatory source.
Independently reviewable – They can be validated in isolation.
Structured content authoring starts with the highest-impact content first—sections that are reused frequently or updated often.
Managing Variants and Localizations
Structured authoring platforms support component variants—localized or adapted versions of a standard content block.
For example:
Global: “Take 1 tablet daily.”
Japan: “Take 1 tablet daily after meals.”
Latin America (Spanish): “Tome una tableta al día.”
These are all variations of the same core component, managed under inheritance models and tagged for geography, language, and product lifecycle stage.
How AI Enhances Component Reuse and Governance
AI-enabled structured content platforms like Docuvera make intelligent reuse easier by:
Recommending components during authoring based on historical usage
Flagging inconsistencies across variants or content types
Suggesting language based on prior approvals
Surfacing reuse opportunities automatically
This not only saves time—it boosts confidence in the consistency and compliance of every document generated.
Structured Authoring Starts with Real, Reusable Content
Structured content isn’t theoretical—it’s operational. It transforms content from static files into governed, traceable assets that accelerate every step of the regulatory process. A content component isn’t code. It’s a paragraph, a statement, a table—treated with the rigor and traceability that regulatory documentation demands.
With platforms like Docuvera, these components become building blocks for submission efficiency: easy to create, easier to manage, and scalable across markets, formats, and geographies.If your content is still locked inside full documents, you’re missing the opportunity to treat it like the asset it is.
Structured content authoring is how you unlock it.