AI-Powered Structured Content Solutions that Reflect How Pharma Works
Purpose-Built for the Use Cases That Matter Most
Pharma teams don’t need more tools—they need the right ones. Most content systems aren’t built for regulated documentation, compliance, or pharma’s digital reality.
Why Others Fall Short
Generic platforms or siloed tools leave critical content—like labeling, filings, SOPs, and medical documents—stuck in static, duplicative workflows.
Docuvera is different
We deliver structured content solutions designed for real-world pharma use. Modular content, controlled variation, and governance come standard—embedded in Centers of Excellence tailored to core use cases across the drug lifecycle.
Explore Solutions by Use Case
Each use case solution brings together template design, workflow logic, and component-based authoring—configurations that support speed without compromising compliance.
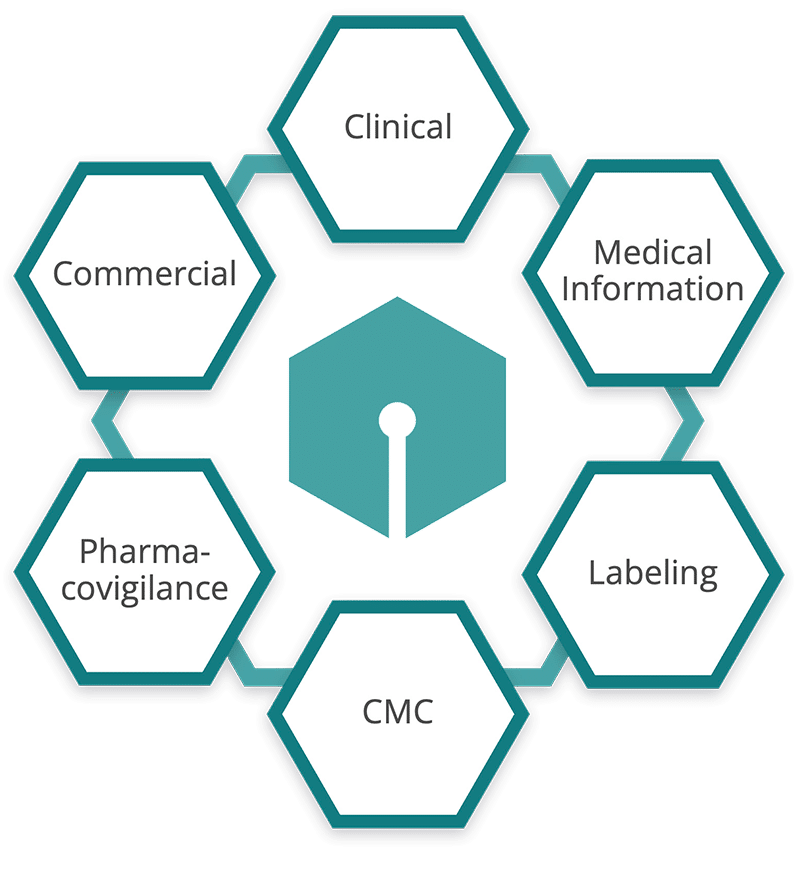
Global Labeling
Structured content for CCDS, SmPC, USPI, and ePI—with support for local and global needs.
Clinical
Standardize protocols, CSRs, and investigator brochures to streamline workflows and reduce rework.
Chemistry, Manufacturing, & Controls (CMC)
Author or update content once and adapt it across Module 3 and site-specific variations.
Medical Information
Manage modular scientific response content with traceability across formats and regions.
Pharmacovigilance
Support recurring safety and regulatory reports with version-controlled components.
Quality & SOPs
Replace static SOPs with structured documentation governed by built-in version control.
A powerful and easy-to-use service in one intuitive platform
The Docuvera platform combines an intuitive web interface with the latest cloud and AI technologies to provide a powerful yet easy-to-use service that relieves the pain of traditional document creation.
Strategic Outcomes that Matter
Built to Serve the Full Content Lifecycle
Docuvera supports the full spectrum of regulated content demands across:

For Regulatory and Compliance Leaders
A governed content foundation that reduces risk exposure, accelerates submission readiness, and ensures traceability across product lines and jurisdictions.

For Content Owners and Medical Writers
A structured authoring environment that simplifies reuse, eliminates version chaos, and reduces back-and-forth in review cycles—freeing teams to focus on accuracy and clarity, not formatting.

For Digital Transformation and Innovation Teams
A scalable content infrastructure that supports modernization without requiring a full system overhaul. Docuvera is designed to complement, not replace, existing RIM, QMS, and publishing systems—unlocking structured reuse while protecting prior investments.
Future-Ready by Design
Docuvera prepares organizations for what’s next—without the guesswork:
As regulatory frameworks continue to evolve, Docuvera ensures your content operations remain aligned—and your teams stay ahead.