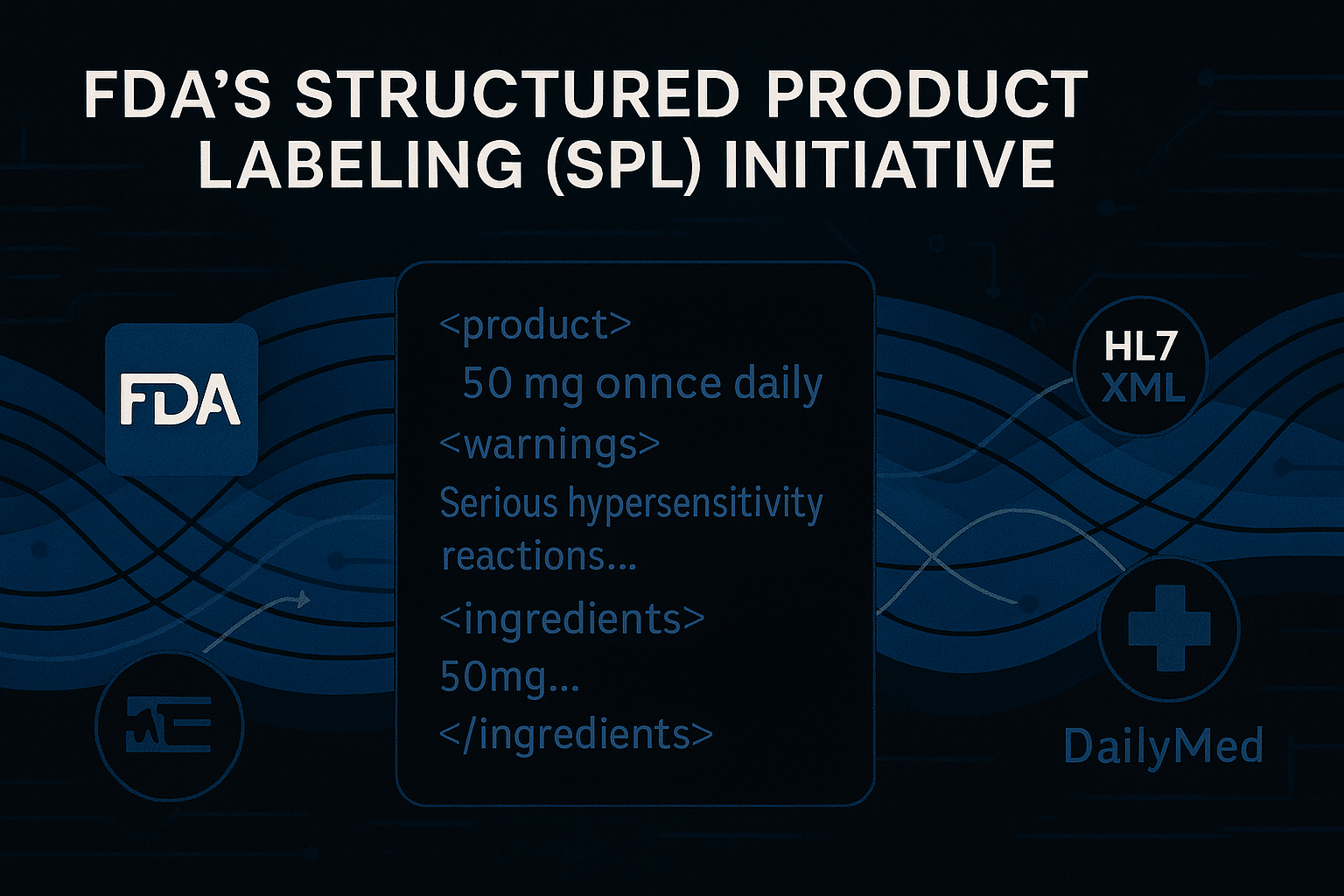
Pharmaceutical Quality–Chemistry, Manufacturing, and Controls (PQ-CMC) is often framed as a technica…
Senior leaders are rarely pulled into submissions because teams are behind schedule. They are pulled…
For Chemistry, Manufacturing, and Controls (CMC) and regulatory leaders, confidence in regulatory ou…
How Regulators Evaluate Change, Why Variations Break, and Where Organizations Stall Without Structur…
Regulatory Work Is Stuck in a Failing Economic Model Pharmaceutical regulatory operations still ru…
A Strategic Overview for Decision Makers, Economic Buyers, and Regulatory Leaders A decision-grad…
For many years, pharma sustainability and regulatory functions ran largely separate agendas: ESG tea…
A Responsible Blueprint for Digital Transformation AI governance is the key to success as pharmac…
This paper makes a clear, urgent case: pharmaceutical digital transformation has stalled because com…
Introduction: Why a Trusted Regulatory Space Is Becoming Necessary Digital transformation in life…
This paper explains how escalating trial complexity, regulatory scrutiny, and interoperability deman…
The 5 Executive Imperatives to Building an Intelligent Clinical Ecosystem is a highly actionable gui…
2026 Will Be the Year Structured Content in Pharma Becomes Essential For years, pharma has managed…
Why Regulatory Teams Are Shifting Toward Modular Content in eCTD 4.0 Submissions Traditional docum…
Introduction: Beyond Technology — The Human Side of Content Transformation in Life Sciences Life s…
Component-Based Labeling is Transforming Pharma Regulatory labeling is a dynamic ecosystem spannin…
The Current State: Siloed Tools and Disconnected Data Flows Regulatory content is everywhere—regul…
Introduction: AI in Regulated Content Management Meets Compliance in Life Sciences Artificial inte…
The Challenge: Fragmented Authoring and Redundant Writing in Clinical Operations For decades, clin…
In the highly regulated world of life sciences, achieving both efficiency and accuracy in content cr…
How governed, structured content enables responsible AI in life sciences. Artificial intelligence…
Global Labeling Harmonization in Pharma: Building a Single Source of Truth Pharmaceutical labeling…
SPOR and IMDP 2.0: Turning Compliance into Competitive Advantage The Identification of Medicinal P…
Digital Twin Pharma: The Future of Regulatory Submissions? Digital twins have transformed industri…
Regulatory Information Management 2025: From Repository to Intelligence Hub For years, Regulatory…
Key Takeaways Structured content in clinical trials enables automation, reuse, and interoperabili…
Breaking Down Silos: Pharma’s Interoperability Imperative Pharma’s digital transformation has is m…
ePI is reshaping pharma’s digital future. Learn how structured content and AI help life sciences com…
eCTD 4.0 is reshaping regulatory submissions for life-sciences. Learn how structured content strateg…
Discover how structured component authoring transforms content creation—driving higher efficiency, t…
Explore how to choose an AI + structured content (AI-SCA) platform for pharma—covering compliance, m…
Discover how AI-powered structured content authoring transforms pharma documentation — from labeling…
Last year I wrote about why booking too far in advance can be dangerous for your business, and this…
Explore how structured content transforms life-sciences: driving reuse, traceability, regulatory rea…
Explore how AI-SCA supports pharma’s shift to digital-first regulation (ePI, IDMP, CTD 4.0) by enabl…
AI on its own isn’t enough. Learn why structured component authoring is the essential groundwork for…
Explore a structured readiness assessment for AI + structured content in pharma—covering leadership…
Why “structured” is the doorway—not the destination—for lifecycle-ready pharma content. Pharma and…
Take a quick self-assessment to benchmark your labeling operations. Learn how structured component a…
Discover why modern labeling demands structured content. Learn how life-sciences companies can build…
Explore how outdated document-centric processes in global pharmaceutical labeling create risk—and ho…
The pharmaceutical industry operates within an increasingly complex regulatory landscape where accur…
Discover why structured content authoring (SCA) is the strategic shift global pharma needs for label…
See how Boehringer Ingelheim accelerated its documentation workflow using structured component autho…
Shift your regulatory submissions from manual to modern with structured content—cut duplication, acc…
In pharmaceutical regulatory operations, most attention goes to visible systems—document management…
Structured content reshapes medical writing—switch from full-document drafting to reusable component…
See what structured content really looks like in pharma—modular, metadata-rich components that drive…
Learn how structured content authoring transforms life-sciences workflows—breaking documents into re…
Explore how structured content authoring accelerates ePI readiness in pharma—driving flexibility, mu…
Explore how modular content and structured component workflows empower pharma companies to optimise…
Traditional CMS tools manage documents; AI-powered structured content platforms manage modular, meta…
A convergence of global mandates, production scale and AI readiness is forcing pharma to adopt struc…
Uncover the truth behind five common myths about AI in pharma documentation—and how structured conte…
Explore 10 essential questions to evaluate AI-powered Structured Content Authoring (SCA) vendors—foc…
Discover how AI-powered structured content authoring transforms pharma documentation — from labeling…
Future-proof your ePI strategy by combining AI-driven structured content authoring with global regul…
Learn how structured authoring transforms ePI from a formatting task into a strategic advantage—enab…
Unlock the power of AI-driven structured content in pharma. This infographic shows how modular, meta…
See how Docuvera combines AI + structured content to transform pharma documentation—one platform for…
AI can boost documentation—but not alone. Explore why pharma must pair AI with structured-content go…
AI raises expectations in pharma documentation—but alone it’s not enough. Learn why structured conte…
There’s a dynamic duo transforming the face of the pharmaceutical industry. Consider the time-consum…
There’s no denying it; pharma is in its innovation era. The life sciences industry is already underg…
Are you wondering where to start with your change management strategy as you integrate Docuvera’s st…
A structured component authoring (SCA) solution can solve challenges for IT departments and provide…
When choosing documentation software for life sciences, IT professionals must keep security, scalabi…
For the life sciences industry, change tracking is integral to the quality assurance process. It all…
Artificial intelligence (AI). It’s everywhere, and it’s changing the world as we know it. From chatb…
Deviating from familiarity can make people feel uncomfortable. At the same time, humans are adaptabl…
Around the globe, artificial intelligence (AI) is all the buzz, including international healthcare e…
Pharmaceutical labeling content requires compliance, accuracy, collaboration between departments and…
In part one of our Pharmaceutical Labeling and the Promise of SCA series, we examined the unique cha…
The drug documentation lifecycle is long and involves many people along the way. From in-depth revie…
Imagine your team of medical information writers has spent days, months, maybe even years authoring…
For many Life Sciences organizations, a leading challenge — on top of a very long product developmen…
In Pharma, one form of technology that offers great promise is a structured component authoring (SCA…
Recently, Docuvera attended DIA Europe 2023, a conference organized by the Drug Information Associat…
For Pharma businesses of all sizes, the right tools can enable efficiency, collaboration and effecti…
Use this checklist to discover if it\'s time to move to a more efficient authoring solution. Structu…
We believe that Life Sciences organizations need a centralized platform for medical information auth…
To keep up with the times or rather, persevere to the future, Life Sciences organizations need to ma…
For Life Sciences organizations, the medical authoring process requires agility to comply with compl…
For Life Sciences organizations, agility in the face of regulatory change is essential. In the compl…
When organizations invest in new platforms and technology, it’s a big deal. From making the actual p…
For authors in the Life Sciences industry, there are typically many moving parts — and many people w…
Medical authoring has long relied on programs like Microsoft Word, and with good reason. The desktop…
Are you still using a program like Microsoft Word for your medical authoring and documentation? What…
When making a decision on a technology vendor, there are many considerations. Will it alleviate a si…
IT teams are the unsung heroes of every enterprise organization. From securing endpoints, maintainin…
Technology can be a game-changer that streamlines complex processes, enhances productivity and incre…
In the Life Sciences industry, anything that can help keep your organization agile in the face of re…
Reliable, compliant documentation is essential, but creating, reviewing, and publishing that content…
On the surface, structured component authoring (SCA) is a concept that makes sense in theory but has…
Imagine this: you’re working on groundbreaking innovations for the Life Sciences industry. Your team…
Docuvera's easy-to-use SCA solution leverages the cloud to provide a streamlined, transparent docume…
Break free from outdated methods in pharmaceutical documentation. Docuvera's Structured Component Au…
With Docuvera and Structured Component Authoring reduce document creation time, increase output, and…
Transform Life Sciences documentation with Structured Component Authoring and streamline workflows…
With cloud-powered SCA revolutionize how you create, review, and publish life science documentation…
Get started
Ready to see what Docuvera can do for you?